The role of substrate roughness in ZnO nanowire (NW) arrays hydrothermal growth has been systematically studied. Six silicon substrates with different roughness by chemical etching have been selected to grow ZnO NW arrays hydrothermally after sputtering 5-nm-thick ZnO seed layer as catalyst. The as-grown samples reveal that average diameters and number densities of ZnO NW arrays are inversely proportional to the increasing substrate roughness observed by atomic-force microscopy and scanning electron microscopy. Furthermore, the theoretically derived equations based on nucleation with the Gibbs free energy to describe relations of substrate roughness versus average NW diameter and NW number density match well with experimental results. Research results in this paper can be used to control the number density and the average diameter of ZnO NW arrays by alternating substrate roughness.
Over decades, zinc oxide (ZnO) nanomaterial has attracted intensive research interests due to its unique mechanical, electrical, chemical, and optical properties [1, 2, 3, 4, 5]. As a wide-band-gap semiconductor, ZnO has various forms in nanostructures like nanoparticles [6], nanoplatelets [7], nanorods [8], nanowires (NWs) [9], nanotubes [10], peculiar nanocastles [11], nanoforests [12], among which ZnO NWs attract special attention in optoelectronics, sensor applications, and nanogenerators [13, 14, 15].
ZnO NWs can be grown on different substrates, such as inorganic [16], organic [17], crystalline [18], and organometallic [19] by physical vapor deposition [20], chemical vapor deposition [21], hydrothermal deposition [22], and pulse laser deposition [23]. Many unique properties and applications of ZnO NWs depend on the NW diameter, number density, or total grown area. It is much more desirable to well control the size, orientation, and density of the NWs for device application. There are some studies on controllable synthesis of ZnO NWs. For instance, the average diameter of ZnO NWs grown by chemical vapor deposition can be controlled by changing the oxygen flow rate and hence the Zn vapor supersaturation [24, 25]. Yang et al. [26] found that ZnO NWs’ position, orientation, diameter, and density could be controlled by epitaxial substrate, the positions of the NWs can be controlled by the initial positions of Au catalyst, and the NW areal density can be controlled by modifying the thickness of Au catalyst. Song et al. [27] found that both the partial pressure of O2 and total system pressure had distinct effects on the final morphologies of ZnO nanostructures.
Some studies have reported the catalyst effects [28, 29, 30]. However, few studies reported the effects of the substrate roughness on ZnO NW arrays growth. However, ZnO NW growth heavily depends on substrate roughness, and such kind of nanostructures also has extensive application in nanodevice fabrication [31, 32]. Besides, comparing with other commonly used synthesis methods, hydrothermal approach takes advantages of lower temperature, easier operation, lower cost, larger-scale production, and more controllability [33]. Thus, it is urgent and extremely important to understand how substrate roughness in hydrothermal synthesis correlates with the ZnO NW growth results. In this paper, we report a detailed investigation on how the diameter and number density of ZnO NW arrays grown by hydrothermal approach vary with substrate roughness.
Six identical silicon substrates were etched by a mixture solution of 0.4 mol/L sodium hydroxide (NaOH) and 26.3 mL isopropyl alcohol (IPA) at 80 ° C for different time [34]. Small pyramids were formed on the silicon surface as a result of anisotropy of wet chemical etching process [35]. Following the general procedure to grow ZnO NWs on the as-etched silicon substrates via hydrothermal method [36], a 5-nm-thick ZnO seed layer was firstly sputtered on all six substrates under the same conditions. In total 50 mmol/L hexamethylenetetramine (HMTA) solution was mixed with 50 mmol/L zinc nitrate hexahydrate in solution at ratio of 1:1 in a beaker. Then seed layer surfaces of substrates were made to float facing down on the 85 ° C liquid solution for 45 min.
We measured the surface roughness of the as-etched substrates before ZnO NW growth by using Park Systems XE-70 atomic-force microscopy (AFM) in non-contact mode. AFM cantilever is ANSCM-PA (Appnano) with resonance frequency of 300 kHz. Field emission scanning electron microscopy (FESEM, JEOL 7000) was employed to characterize the morphology of the as-grown ZnO NWs.
The morphologies of the six substrates with different roughness are shown in Fig. 1. The average roughness of the six substrate surfaces was measured to be 32.0 ± 1.6, 66.3 ± 3.3, 103.9 ± 5.2, 221.0 ± 11.0, 238.1 ± 12.0, and 256.0 ± 12.8 nm, respectively (Fig. 1a-f). Substrate roughness was obtained by three times measurements on every substrate with randomly selected area of 30 μ m × 30 μ m, and the multiple measurements could significantly reduce radom errors. An extremely thin ZnO seed layer was deposited on all etched substrates for facilitating ZnO NW growth [37]; on the other hand, since our etched samples have relatively high roughness from about 32-256 nm compared to 5-nm-thick ZnO thin film coverage, the influence of seed layer can be reasonably ignored.
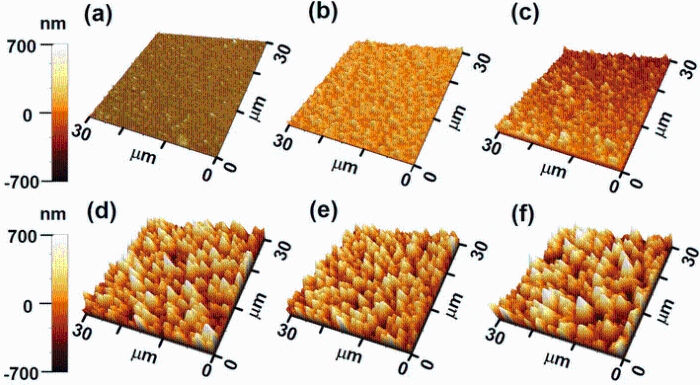 | Fig. 1 AFM measurement for six substrates with different roughness: a 32.0 ± 1.6 nm, b 66.3 ± 3.3 nm, c103.9 ± 5.2 nm, d 221.0 ± 11.0 nm, e 238.1 ± 12.0 nm, f 256.0 ± 12.8 nm |
Figure 2a-f shows the surface morphologies of all six samples in an area of 1500 nm × 1135 nm, exhibiting the clear hexagonal shape of ZnO NWs with wurtzite structure and also demonstrating a variation in the morphology upon increasing substrate roughness.
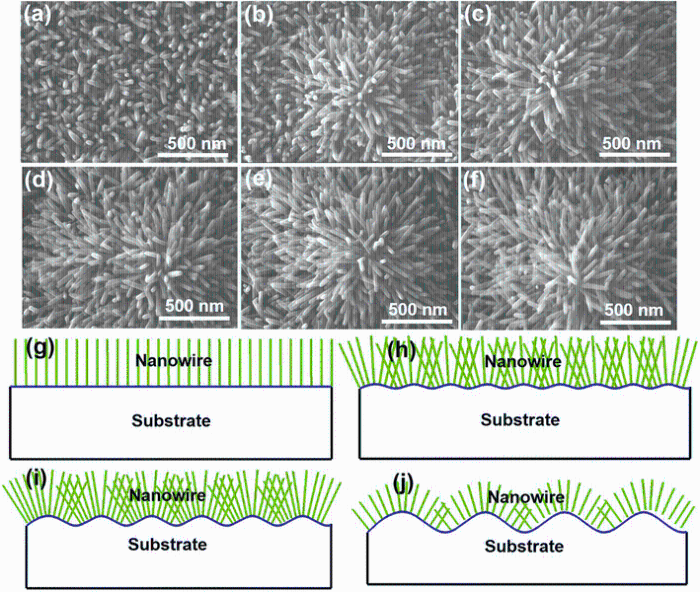 | Fig. 2 a-f SEM images of the as-grown NW arrays on substrates presented in Fig. 1, accordingly, g-j schematic diagram demonstrating how morphology and orientation of ZnO NWs evolve with substrate roughness from flat to coarse |
The interpretative schematic diagram in Fig. 2g-j demonstrates the growth evolution— how the morphology and orientation of NWs change with increasing substrate roughness. It can be observed that morphology of NWs varies distinctly from almost vertical alignment to flower-like along with the roughness increase. In addition, despite the difference in substrate roughness, all individual NWs are approximately perpendicular to the local surface of the substrate rather than in the normal direction of the entire substrate.
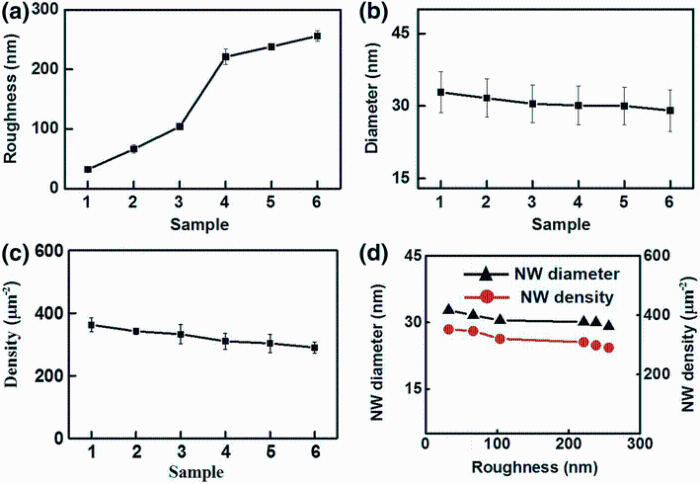
In order to statistically analyze the average diameter of NWs and number density of NWs, we used the statistical software named Nano Measurer with high accuracy to analyze all six samples based on their top-view SEM images with the same magnification and pixel size. Figure 3a shows the roughness distribution among those samples. It can be seen that the average diameter with standard deviation about 13% decreases slightly with increasing substrate roughness, so does the number density of NWs with standard deviation about 6%, which is the number of NWs in the specific area as shown in Fig. 2. The average diameter of NWs for six samples varies from 29.1 to 32.8 nm, and the number density ranges between 290.3 and 352.3 μ m-2 as shown in Fig. 3b, c. Moreover, as shown in Fig. 3d, both averaged NW diameter and NW number density decrease with the growing roughness. All statistics data are summarized in Table 1.
| Table 1 Substrate parameters and NW statistic results |
In order to explain the experimental results, the classic nucleation theory was developed to study the relationship between substrate roughness, average diameter, and number density of the NWs. Referring to Wenzel model [38], we can define the roughness factor r as the ratio of the surface area S r of a factual rough substrate to a smooth substrate with surface area S0 based on the fact that the surface area S r of a factual rough substrate increases with its surface roughness value. So the roughness factor r of a factual substrate surface can be written as:
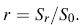
Furthermore, since the intrinsic contact angle θ is designated by the wetting angle of solution on the smooth surface, wetting angle θ * of rough interface can be expressed as [39]:
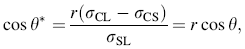
where σ CL, σ CS, and σ SL are the specific crystal/liquid interface energy, crystal/substrate interface energy, and substrate/liquid interface energy, respectively.
According to Eq. (2), wetting angle of rough surface will decrease along with increasing roughness factor if the initial contact angle on flat substrate is less than 90° . While in real chemical reaction, the initial nucleation happens on interface of solution droplet and substrate, and the water contact angle was measured to be about 38° on freshly sputtered ZnO seed layers on Si substrate [40]. Thus, we can reasonably assume an expression of surface free energy with roughness factor by using classic Young’ s equation [41]:
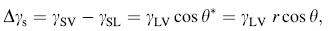
where γ SV, γ SL, and γ LV indicate the solid-vapor, the solid-liquid, and the liquid-vapor interfaces energy, respectively.
According to nucleation theory, nucleation density has the following equation [42]:
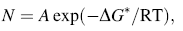
where N is nucleation density, ∆ G* is activation energy of nucleation, which contains volume free energy ∆ GV and surface free energy ∆ GS or ∆ γ S, T is growth temperature, and A is a constant.
Therefore, we can combine Eq. (3) and Eq. (4) together to achieve an expression of nucleation density, which is linked to surface roughness or surface free energy as:
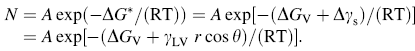
It is noticeable that increased roughness factor r will firstly increase the Gibbs free energy change (∆ G* ) by increasing free surface energy and then decrease the probability of nucleation sites density, which results in diminishing of NW number density. From the statistical analysis of the ZnO NW sample synthesized with different substrate roughness (Table 1), when substrate roughness linearly increased from 32.0 to 256 nm, NW number density accordingly decreased from 352.3 to 290.3 μ m-2, which is in accordance with Eq. (5).
Meanwhile, based on the literature that quantitatively describes controllable diameter of ZnO NW growth from classical nucleation theory, the diameter of ZnO NW in this experiment can be written as [24]:
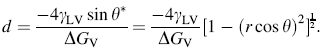
Although the sizes of ZnO NW synthesized by wet chemical method are strongly dependent on Zn2+concentration [43], the effect from substrate roughness is still distinguishable. From Eq. (6), we can infer that the diameter of ZnO NW will be slightly and reversely proportional to the substrate roughness, which matches well with our experimental observations that sizes of ZnO NWs drop from about 33 nm down to about 29 nm proportionally with increasing substrate roughness.
The effects of substrate roughness on the morphology of ZnO NW arrays grown via hydrothermal method were systematically investigated. Six etched silicon substrates with different roughness were purposely prepared for growing ZnO NW arrays. The substrate roughness and the as-grown NW samples were characterized by AFM and SEM, correspondingly. Statistical analysis showed that average NW diameter and number density of ZnO NW arrays were inversely proportional to the increase in substrate roughness. To interpret this result, two equations to describe number density roughness and average diameter roughness based on classic theory of nucleation and the Gibbs adsorption equation were successfully deduced, respectively. Those revealed findings are important for understanding the key role of substrate roughness in NW growth and to further help to well control the growth of ZnO NWs for high-performance nanodevices.
The authors specially thank Prof. Mankey for sputtering the ZnO film. This work has been supported by Jiangsu Provincial Department of Education with the 2012 Project of Overseas Research of Distinguished Young and Middle-aged Teachers and Principals, the University of Alabama startup fund.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|