For corrosion protection of carbon steel in a marine environment, cold arc thermal spray coating was applied to the surface with Al and Al-Mg alloy wires. The surface hardness of Al and Al-Mg thermal spray coatings increased with Mg content. And the various electrochemical experiments were carried out to evaluate corrosion damage characteristics of the thermal spray coating layers. The Al and Al-Mg thermal spray coating layers presented negative potentials compared to carbon steel in corrosion potential measurements. And an anodic polarization experiment revealed a tendency of activation polarization with no passivation. Furthermore, the corrosion damage of the thermal spray coating layer in galvanostatic experiment was observed mainly at the defect area, and the Al-3Mg thermal spray coating layer presented less surface damages than others. In addition, the Al-3Mg thermal spray coating layer showed the lowest corrosion rate while having a sufficient driving voltage for cathodic corrosion protection. Therefore, it is an optimal thermal spray material for sacrificial anode.
Various surface treatment techniques are applied for corrosion protection of the steel in the marine environment. In particular, thermal spray coating is useful for the long life of large structures such as bridges, ships, and offshore plants[1, 2, 3]. Thermal spray coating is a technology for forming a film by heated thermal spray material which is in a melted or semi-molten state, spraying the particles to the substrate at high speed, which is then collided and deposited to form a thin film. In general, the arc thermal spray method is mainly used for corrosion protection in the seawater environment because it has economic advantages, high productivity, and excellent field constructability for large complex steel structures compared to other thermal spray methods [4]. Al, Zn, and Al-Zn thermal spray wires are mainly used for thermal spray coating materials. In particular, Zn-Al thermal spray coatings with the flame and arc spraying methods have been widely used for the long-term corrosion protection of the structural steel due to synergy effect of the great galvanic protection of Zn and the satisfactory erosion resistance of Al[5, 6]. However, in this paper, Al-Mg thermal spray coating is applied to SS400 steel for the enhancement of the erosion resistance. Since Al-Mg alloys (5xxx series) have excellent characteristics such as low density, good mechanical properties, and better corrosion resistance, they have been commonly used in marine applications such as shipbuilding and coastal equipment [7, 8]. The mechanical properties of Al-Mg alloys increase with Mg content via solid solution hardening [9]. In general, the Mg content in the commercial wrought alloys rarely exceeds 5% Mg because the increased susceptibility to pitting corrosion is related to the high concentration of Mg content [10]. Mg in aluminum precipitates at the grain boundaries as soon as the temperature becomes lower than the solidus temperature. This reason is that the solubility of Mg is very high at elevated temperature, about 15% at 450 ° C, but it dose not exceed 1% at the room temperature[10]. The intermetallic particles in Al-Mg alloys include mainly the β -phase (Al3Mg2 or Al8Mg5) which can be the action as anodes to the aluminum matrix and become the common sites for pitting nucleation [8, 9, 10, 11].
In this paper, Al-Mg thermal spraying was conducted for corrosion protection of the steel. These kinds of thermal spray coatings show protective characteristics of sacrificial anode maintaining a negative potential compared to the substrate. They are oxidized first in the seawater environment, which supplies electrons to the substrate, and corrosion protection is achieved by reduction reaction. The corrosion protection performance of the thermal spray coating layer is important for the extension of the life of the steel structures and their safety. Therefore, the various electrochemical characteristics of Al and Al-Mg thermal spray coating layers were investigated in the natural seawater environment in this study.
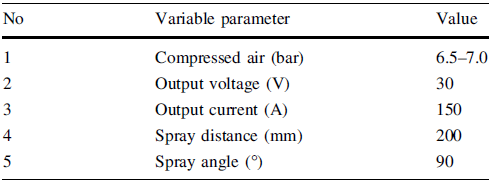
The popular structural steel SS400 was used as the substrate specimen. For surface treatment, a rough surface was formed by grit blasting. Arc thermal spraying method was used for this experiment, with a robot control system to maintain constant spray conditions of the spray gun. The spray work was performed with Al, Al-3Mg, and Al-5Mg alloy wires. The spraying parameters are shown in Table 1. The SS400 specimen that was not thermal spray-coated was polished with sand paper No. 2000, and then an electrochemical experiment is carried out under the same conditions as the thermal spray-coated specimens. Every electrochemical experiment was performed in the natural seawater environment with an exposure of the area of 0.332 cm2 with Ag/AgCl as a reference electrode and the platinum as a counter electrode. The corrosion potential measurement was taken for 72 h, and the initial stabilization time for polarization experiment was set to 3600 s. The anodic and cathodic polarization experiments were performed in the open-circuit potential (OCP) at the scan rate of 2 mV/s up to +4.0 and -3.0 V, respectively. In addition, after polarization to ± 0.25 V at the OCP, the corrosion current density and corrosion potential were determined by Tafel’ s extrapolation method. The galvanostatic experiment was performed for 3600 s at the current density of 1 × 10-4, 1 × 10-3, and 1 × 10-2 A/cm2. After the anodic polarization and galvanostatic experiments, the surface damage morphologies were observed by scanning electron microscope (SEM) and 3D microscope. And the micro-Vickers hardness of the thermal spray coating layer was measured more than 10 times, and their average values were determined.
| Table 1 Arc thermal spraying parameters |
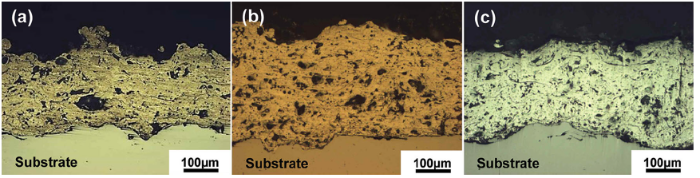
Figure 1 presents cross-sectional morphologies of Al and Al-Mg thermal sprayed coating layers. The results of coating properties are tabulated in Table 2. For Al and Al-Mg thermal spray coatings, the coating thicknesses were about 220-290 μ m and the overall porosity percentages were around 7-12%. Al-3Mg coating was relatively thicker having higher porosity than those of others. The micro-Vickers hardnesses of Al-Mg coating were higher than that of Al coating and increase with increasing Mg content. Although the hardness of Al-3Mg was relatively higher than that of Al coating, the mechanical properties become generally worse with increasing their porosity. It is well known that the mechanical properties of Al-Mg alloys are improved with Mg content[9]. On the other hand, it is believed that arc thermal effects during the thermal spray coating process are almost never influenced on their mechanical properties. The reason is that Al-Mg alloys do not attribute to age hardening, and their strength is from the combination of work hardening and solid solution strengthening[10, 12, 13].
| Table 2 Coating properties of Al and Al-Mg thermal spray coating layers |
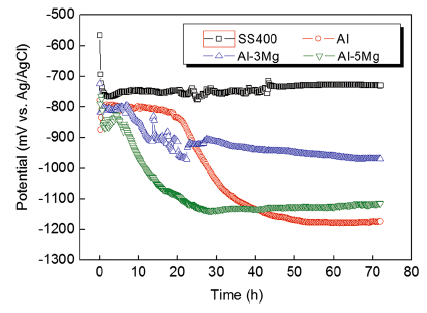
Figure 2 shows the corrosion potential measurements of SS400 (substrate) as well as the Al and Al-Mg alloy spray-coated specimens for 72 h in a natural seawater solution. At the beginning of immersion, the potentials of Al and Al-Mg spray-coated layers quickly moved in the active direction. This stage corresponds to the process of solution filling or penetrating into the pores and cracks on the coating surface [14]. After this period, the potentials of Al and Al-Mg spray-coated layers presented a similar tendency which moves to the active direction after a certain time of immersion. It seems that the passivation film on the Al surface that was formed during the early stage of immersion was destroyed by the Cl- ions in the seawater and the potential moved in the active direction as a result. It is known that formation of Al2O3 passive film is likely after the immersion of the Al-based coatings in the artificial seawater, which can be explained by the following reaction: 2Al + 3H2O → Al2O3 + 6H+ + 6e. Al2O3 film is not stable in water and tends to change to Al(OH)3. Accumulation of the oxide/hydroxide film on the electrode surface diminishes dissolution of Al. However, the presence of chloride easily triggers the breakdown of this film by the interaction between Al(OH)3 film and Cl- ions, which produces AlCl3 after sufficiently long time[15, 16]. Hence, within 30 h of immersion time, potential fluctuations of Al-Mg spray-coated layers were observed due to the simultaneous formation and destruction of the passivation film by the Cl- ions in seawater [17]. After 42 h of immersion, the Al-Mg spray-coated layers showed nobler potentials than that of the Al spray-coated layer even though they contained Mg which has a large active potential. This seems to be due to the multilayered surface of the spray-coated layer that has micro-defects. According to other researchers, the pure Al with surface defects formed a lower corrosion potential than the fine polished pure Al [18]. Furthermore, in the case of Al and Al-Mg alloys, crevice corrosion led to sensitive changes in the corrosion potential by the changing pH [18]. On the other hand, the SS400 steel to be protected showed a noble potential of -560 mV as soon as it was immersed and then the potential quickly moved in the active direction. At around 1 h of immersion, it showed an active potential of -770 mV and then slowly moved in the noble direction in general. At the end of the experiment, the potential of -730 mV was measured. Typically, it is regarded that there is an effect of corrosion protection by sacrificial anode when a low potential difference that is lower by 50 mV or more compared to the substrate, and the national association of corrosion engineers (NACE) specifies a potential difference of minimum 100 mV [19]. Therefore, in order for the substrate to be protected by the sacrificial anode principle in this study, an active potential of minimum -870 mV must be maintained. The potential difference in the spray-coated layers compared to the substrate SS400 at the end of the experiment was 440 mV for Al, 380 mV for Al-5Mg, and 240 mV for Al-3Mg in descending order. The corrosion protection effects for the substrate will appear because sufficient driving voltage will be generated for the substrate [20].
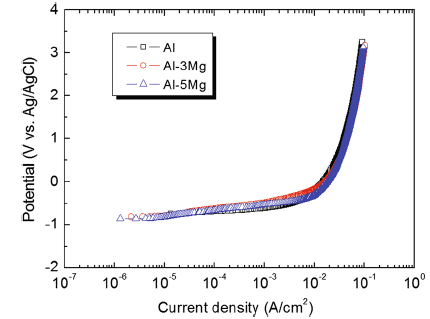
Figure 3 presents anodic polarization trends of the Al and Al-Mg alloy spray-coated specimens in a natural seawater solution. The current density generally increased with the rising potential for all specimens. In particular, as the potential increased from the open-circuit potential to 0 V, the current density sharply increased until around 1 × 10-2 A/cm2. After 0 V, the current density slowly increased as the potential increased until the end of the experiment. Furthermore, all specimens showed activation polarization with no passivation characteristics. The reason for this seems to be the combined effects of the formation of unstable films due to such process factors as air impurities included in the spray coating work, partially formed oxides, and rough surface condition, as well as the destruction of the films by Cl- ion during the initial stabilization before the polarization experiment. Some aluminum alloys formed Al2O3or Al2O3· 3H2O on their surfaces in the seawater environment and exhibited excellent corrosion resistance due to the passivation characteristics where the current density partially decreased [17]. In the case of sacrificial anode, however, no passivation film must be formed in order to promote a uniform corrosion throughout the surface and to prevent the generation of local corrosions such as pitting [21]. Therefore, all the thermal spray-coated layers used in this experiment showed ideal sacrificial anodic behavior.
Figure 4 depicts surface morphologies captured by SEM and 3D microscope on Al and Al-Mg alloy spray-coated specimens before and after the anodic polarization experiments. In Fig. 4a, thin splats were observed on the as-sprayed coating surface resulting from the collision of droplets melted by arc heat at high speed. The edge of splat was uplifted because when the spray particles collide, the thin splat did not strongly attach to the substrate and the internal compressive stress during the coagulation was relatively lower than that at the center [22]. In the case of the Al spray coating layer, pore defects were generated on the splat. When the molten droplets including isolated gases are deposited on the substrate and the isolated gases evaporate during the following coagulation process, many pore defects were formed on the adhered splats [23]. After the anodic polarization experiment, surface damages of spray-coated specimens exhibited a similar tendency leading to the active dissolution reaction throughout the surfaces. In particular, they were divided into splat areas where it was relatively dark and splat-surrounding areas that appeared bright due to active dissolution reaction. Considering the pattern of corrosion damages around the splats, the active dissolution reaction appears preferentially at the edges of the splats and progresses to the center. Therefore, the exposed surface area of the spray-coated layer increases with the progress of the active dissolution reaction because the spray-coated layer consists of a lamellar structure. The active dissolution reaction occurs from the outermost splats of the spray coating layer, and with the reduction in their surface area, the laminated splats at the bottom sequentially participate in the active dissolution reaction, increasing the surface roughness after this experiment. This can be verified through 3D analysis from the fact that the surface damage depth generally increased after the anodic polarization experiment in Fig. 4b. When the deviations of the surface damage depth before and after the anodic polarization experiment were compared, Al showed the greatest deviation followed by Al-5Mg and Al-3Mg, and their values were 65.3, 37.2 and 31.0 μ m, respectively. Consequently, the Al-3Mg spray coating layer not only showed the smallest deviation of surface damage depths, but its surface damage was of the uniform corrosion type, indicating a damage tendency that is most appropriate as the spray coating material with sacrificial anode function for corrosion protection. However, these results are limited to the anodic polarization experiment, and more comprehensive analyses are required.
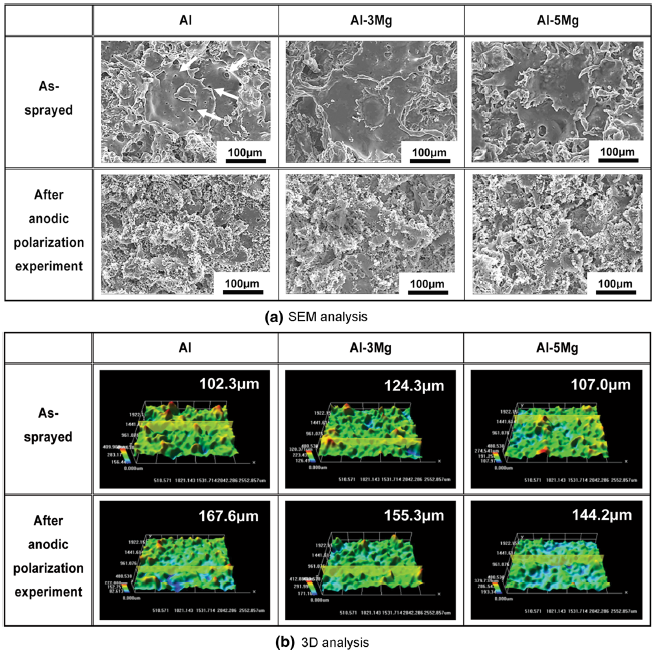 | Fig. 4 Surface morphologies of thermal sprayed coating layers after anodic polarization experiment in seawater: a SEM analysis, b 3D analysis |
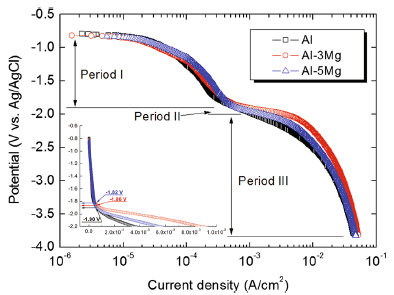
Figure 5 shows cathodic polarization trends of Al and Al-Mg alloy spray-coated specimens in the natural seawater solution. All cathodic polarization curves presented similar tendency with decreasing potential, indicating that the cathodic reaction process goes through three stages as follows [24].
I: Concentration polarization was observed by the reduction reaction of dissolved oxygen (O2 + 2H2O + 4e → 4OH-) in the early stage of polarization.
II: The current density sharply increased around -1.85 V, which is the turning point, due to the acceleration of activation polarization by hydrogen evolution (2H+ + 2e → H2) at the coating surface. It has three major steps. First, H+ reacts with an electron from the coating, forming an adsorbed hydrogen atom, Hads (H++ e → Hads). Two of these adsorbed atoms react in second step to form a hydrogen molecule (Hads + Hads → H2). In a third step, hydrogen molecules are sufficiently combined and hydrogen bubbles were generated on the coating surface. As shown in Fig. 5, the turning points of Al, Al-3Mg, and Al-5Mg were measured at -1.90, -1.86, and -1.82 V, respectively.
III: The reduction of water leads to generation of hydrogen gas (2H2O + 2e → H2 + 2OH-) with more negative cathodic potentials. Therefore, we can observe white calcareous deposits on the coating surface. The reduction reaction of dissolved oxygen and water generates OH- ions near the coating surface resulting in the formation of calcareous deposits on the coating surface. It is known that the calcareous deposits are mixtures of CaCO3 and Mg(OH)2[25, 26]. The mechanism for the formation of calcareous deposits [26, 27] is believed to be (1) Mg2++ 2OH- → Mg(OH)2↓ , (2) HCO3- + OH- ↔ H2O + CO32-, (3) Ca2+ + CO32- → CaCO3↓ . The formed compact calcareous deposits can act as a physical barrier against oxygen diffusion and thus decreases the current density, or the sacrificial anode consumption, keeping efficient protection [26, 28]. However, it is difficult to expect the great effect of corrosion protection in this experiment because calcareous deposits did not form a compacted and thick barrier on the coating surface. The reason is that the time for forming the calcareous deposits is too insufficient.
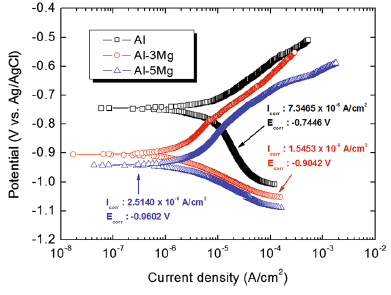
Figure 6 depicts polarization curves for Tafel analysis of Al and Al-Mg alloy spray coating layers in natural seawater solution. The corrosion current density (Icorr) and corrosion potential (Ecorr) were obtained through the Tafel’ s extrapolation method. The corrosion current density was the lowest in Al-3Mg, followed by Al-5Mg and Al. The greatest corrosion current density of Al is believed to be due to coarse pores and cracks that provide the paths for seawater to penetrate to the substrate SS400 as shown in Fig. 1[29]. In addition, the corrosion rate of the coating layer is accelerated because Al spray coating forms a galvanic cell with the SS400 which shows a nobler potential [30]. On the other hand, Al-3Mg coating layer showed that the corrosion current density is the lowest in spite of the greatest porosity in its coating layer. The reason is that Al-3Mg spray coating has smaller size pores and thicker coating layer than that of Al spray coating. However, Al-5Mg spray coating presented greater corrosion current density than that of Al-3Mg spray coating despite relatively low porosity. It is probably because the chemical composition can play a more significant role than porosity in corrosion activity [31]. Al-Mg alloys with Mg content greater than ~3.5 wt% become supersaturated at temperatures lower than ~200 ° C [32], and this can lead to the deleterious precipitation of β -phase (Al3Mg2 or Al8Mg5) which is anodic with Al-Mg alloy matrix and promote rapid pitting corrosion through galvanic interaction [33]. The corrosion potential was the most negative in the Al-5Mg spray coating layer, followed by Al-3Mg and Al. Cathodic corrosion protection typically has better corrosion protection effect when the potential of the sacrificial anode is lower, and the efficiency of the sacrificial anode becomes greater as the corrosion rate is lower [20]. Therefore, the Al-3Mg coating layer is the most optimal spray coating material for sacrificial anode because it showed the lowest corrosion rate having a sufficient driving voltage for cathodic corrosion protection.
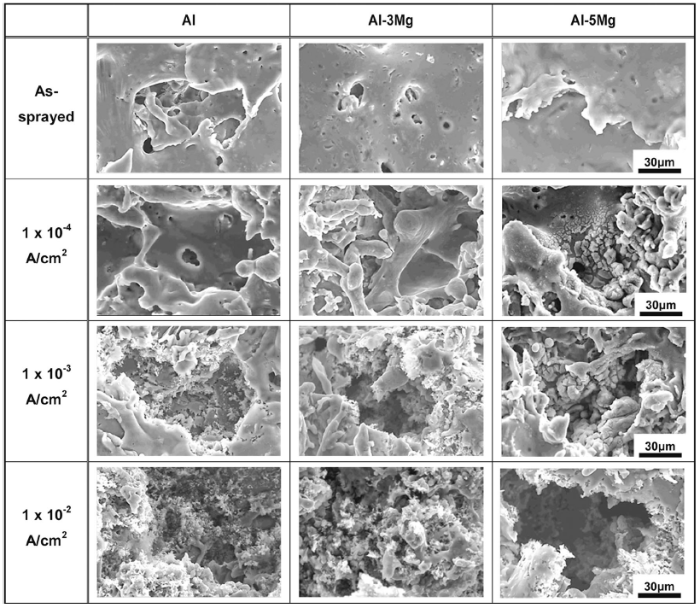
Figure 7 describes surface damage morphologies of Al and Al-Mg alloy spray-coated specimens after galvanostatic experiments for 3600 s in natural seawater solutions. First, molten droplets were laminated as splats on the surface of the spray coating layer before the experiment, and local sunken defects were observed which formed during the spray coating process. In the case of the Al spray coating layer, corrosion damage by anodic dissolution reaction was barely observed at 1 × 10-4 A/cm2, whereas the inside of local sunken defects were generated at 1 × 10-3 A/cm2. The anodic dissolution reaction inside the defects on the coating surface occurred more intensively at 1 × 10-3 A/cm2, leading to greater corrosion damage. This phenomenon occurred around the defect sites of the spray coating layer that consisted of a multilayer structure because the Cl- and H+ ions were concentrated inside the defects as a consequence of reduction reaction. Hence, low pH atmosphere was formed inside the defects, which accelerated the corrosion damage [20, 34, 35]. At 1 × 10-2 A/cm2, broad sunken surface damage was observed on the coating layer. The reason for this seems to be the detachment of part of the incompletely laminated coating layers during the spray coating process, and corrosion damage due to dissolution reaction was observed inside these defects as well. Next, in the case of the Al-3Mg spray coating layer, surface damage was generally fewer than that of the Al spray coating layer. First, at 1 × 10-4 A/cm2, no corrosion damage by dissolution reaction was observed throughout the surface as well as inside the defects, indicating better corrosion resistance than the Al spray coating layer. However, at 1 × 10-3 A/cm2, corrosion damage by anodic dissolution reaction was observed in the defect sites of the spray coating layer, but sites with no corrosion also existed. At 1 × 10-2 A/cm2, the surface damage tendency which was detached by the anodic dissolution reaction was similar to that of the Al spray coating layer. In Al-5Mg spray coating layer, the corrosion products were generated by anodic dissolution reaction inside the defects in the spray coating layer at 1 × 10-4 and 1 × 10-3 A/cm2, and the corrosion products increased with applied current density. However, corrosion damage showed a smaller overall surface damage area compared to the Al spray coating layer. On the other hand, at 1 × 10-2 A/cm2, the tendency of local corrosion damage was observed on the coating surface along the depth. The difference in local corrosion resistance of Al, Al-3Mg, and Al-5Mg is related to the difference in intermetallic particle characteristics present. Ezuber et al.[8]and Aballe et al.[36] studied the corrosion of AA1050/AA1100 and AA5083 in 3.5% NaCl solution and natural seawater. They observed a similar tendency in potentiodynamic polarization curves. The lager local corrosion damage of AA5083 was contributed to the higher number of cathodic intermetallic particles. In this study, the Al-5Mg alloy wires have enough Mg content to contribute to supersaturation of the element and leads to solid solution strengthening. However, this concentration also forms an abundance of the β -phases (Al3Mg2, Al8Mg5, Mg2Al3) which are known to be deleterious in the corrosion performance. Because the β -phase is anodic, its presence enhances the selective dissolution on the coating surface, thus creating sites for deep pits in seawater [8, 9, 10, 11, 33]. The local corrosion type of Al-5Mg spray coating layer lowers the efficiency of sacrificial anode and will have the negative effect on the sacrificial anode for corrosion protection [37].
1.The surface hardness of Al and Al-Mg thermal spray coatings increased with Mg content. Therefore, the hardness of Al-5Mg thermal spray coating was up to about 49% larger than that of Al thermal spray coating.
2.Corrosion potential measurement suggested that all the spray coating layers exhibit corrosion protection effects by the sacrificial anode principle for the substrate SS400.
3.In the anodic polarization experiment, no passivation characteristics appeared in all the spray coating layers and ideal activation polarization behaviors were observed as sacrificial anode materials. The Al and Al-Mg alloy thermal spray coating layers exhibited corrosion damage throughout the surface, but the surface damage depth tended to increase a little.
4.The Al and Al-Mg alloy thermal spray coating layers at the cathodic polarization experiment presented similar trends in concentration and activation polarizations.
5.At the galvanostatic experiments, the Al-3Mg spray coating layer presented excellent surface damage behavior of the spray coating layers due to a uniform corrosion damage trend.
6.The Al-3Mg spray coating layer was the most optimal spray coating material for sacrificial anode because it showed the lowest corrosion rate having a sufficient driving voltage for cathodic corrosion protection.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|