The microstructural evolution and tensile properties of Ti-3Al-8V-6Cr-4Mo-xZr (x = 0, 2, 4 and 6) titanium alloys were investigated. The precipitated phases and tensile fracture morphologies were observed using scanning electron microscopy and transmission electron microscopy. Experimental results show that the presence of trace impurity Si and the addition of Zr induce the formation of (TiZr)6Si3 silicides. The quantity of silicides increases with Zr content increasing. The dispersed silicides refined the grain size of β Zr-containing alloys, and the grain size decreases significantly with Zr content increasing. Accompanying these microstructural changes, the strength of the alloys is enhanced gradually with the increase of Zr content, which is attributed to the combination of precipitation strengthening and grain refinement.
Metastable β titanium alloys exhibit high specific strength, fatigue strength, corrosion resistance and favorable room temperature formability [1, 2, 3]. Therefore, they are increasingly considered as candidates to replace high-strength steels and Al-base alloys in aerospace field, in order to reduce the weight and increase the fuel efficiency of air/space vehicles. Over the years, considerable efforts have been devoted to the investigation of microstructures, phase transformation and mechanical properties of these alloys [4, 5, 6], and some new metastable β titanium alloys have been developed [7, 8]. Most of the high-strength metastable β titanium alloys are based on the Ti-Al-V-Cr-Mo system, such as Ti-5Al-5Mo-5V-3Cr (Ti-5553)[3, 9], Ti-5Al-5V-5Mo-1Cr-1Fe (VT22) [10], Ti-3Al-8V-6Cr-4Mo-4Zr (Beta C)[11] and Ti-3.5Al-5Mo-6V-3Cr-2Sn-0.5Fe[12]. However, rapid β grain growth is still an unsolved problem for β titanium alloys, when they are heat-treated in β phase field. Among the above, Beta C alloy exhibits less tendency of β grain growth, but the reason for this has not been clarified up to now.
Zirconium is an important neutral alloying element in titanium alloys, which is usually added to Si-bearing near α high-temperature titanium alloys, such as Ti-6Al-5Zr-0.25Si-0.5Mo (IMI 685) [13], Ti-5.5Al-3.5Sn-3Zr-1Nb-0.3Si-0.25Mo (IMI 829) [14]and Ti-6Al-4Sn-4Zr-0.7Nb-0.4Si-0.5Mo (IMI 834) [15]. On one hand, Zr enhances the strength of high-temperature titanium alloys; on the other hand, Zr can dramatically decrease the solubility of Si in titanium and induce the formation of dispersed silicides, which play a role in improving the creep performances by pinning mobile dislocations. However, there are no systematic investigation to be reported on the effects of Zr content on the microstructures and mechanical properties of Ti-3Al-8V-6Cr-4Mo-xZr (x = 0, 2, 4 and 6, in wt%) alloys (abbreviated as Ti-3864x). Such investigations are instrumental to explore whether the silicides precipitated in Ti-3864x alloys due to the addition of Zr, or how are they formed and realize the β grain refinement.
The chemical compositions of the four alloys used in this study are listed in Table 1. Silicon is an impurity from the raw materials in these titanium alloys. The β transus temperature of these alloys is about 740 ° C measured by metallographic technique. The rolled bars of 12 mm in diameter were obtained by conventional hot rolling processing. Samples for microstructure observation and tensile tests from the as-rolled bars were solution-treated at 800 ° C for 1 h and then water-quenched to room temperature.
| Table 1 Chemical composition of the alloys (in wt%) |
The microstructures of the alloys were investigated by an optical microscope (OM, ZEISS Axiovert 200MAT) and a scanning electron microscope (SEM, ShimadzuSSX-550) with an energy-dispersive X-ray (EDX) detector. SEM observation for silicide precipitates was carried out in the backscattered electron (BSE) mode, and the polished samples were not etched. Image-Pro Plus software was utilized for the grain-size statistics. The specimens for transmission electron microscope (TEM) observation were manually ground to 50 μ m thick, punched into disks of 3 mm in diameter, and then ion-milled to electron transparency in a Gatan ion miller. TEM observation was carried out on a Tecnai G220 TEM with an energy-dispersive spectroscopy (EDS).
Tensile tests at room temperature were performed on an Instron 5582 testing machine at a constant cross-head speed of 1 mm/min. Tensile specimens with a gage diameter of 5 mm and gage length of 30 mm were used. The fracture surfaces were observed on SEM.
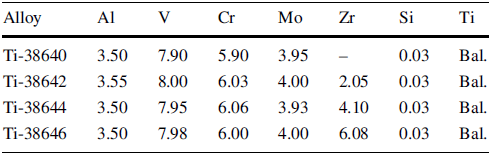
Figure 1 shows the optical microstructures and grain-size distribution of the Ti-3864x alloys after solution-treated at 800 ° C for 1 h. The microstructures are comprised of equiaxed β grains. The average β grain size decreases gradually as the compositions change from Ti-38640 to Ti-38646, implying that the grain sizes depend on Zr content. Furthermore, the grain-size distributions of Ti-3864x alloys are markedly different. The higher the Zr content, the narrower the grain-size range, as shown in Fig. 1e-h. The grain size of Ti-38640 alloy ranges from 15 to 150 μ m, with an average value of 55 μ m (Fig. 1e). The average grain size of Ti-38642 alloy is 39 μ m, and the maximum grain size is about 110 μ m (Fig. 1f). As shown in Fig. 1g and h, the average grain sizes of T-38644 and Ti-38646 alloys are 33 μ m and 26 μ m, respectively. The maximum grain size is 90 to 55 μ m, respectively. Furthermore, over 97% of the grains are in the size range between 15 and 40 μ m in Ti-48646 alloy. The above analysis indicates that the grain size of Ti-3864x alloys becomes more uniform with the increase of Zr content.
 | Fig. 1 Microstructures and grain-size distribution histograms of solution-treated Ti-3864x alloys: a, e Ti-38640; b, f Ti-38642; c, g Ti-38644; d, h Ti-38646 |
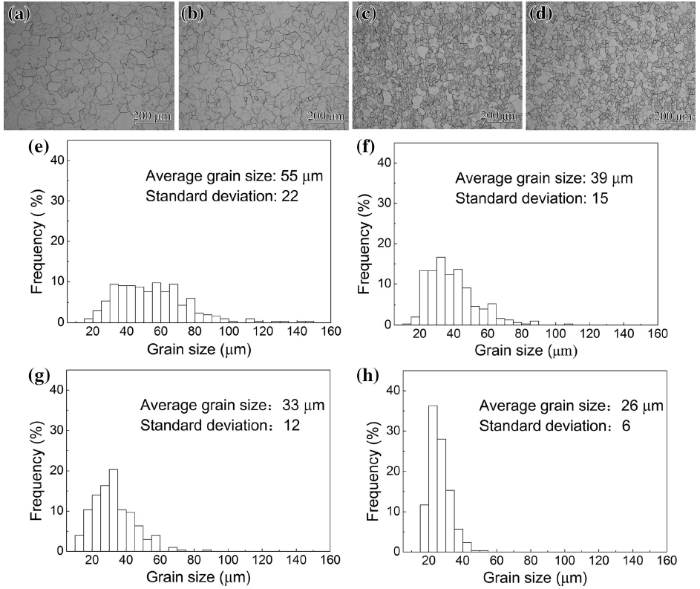
Figure 2 shows the BSE images and EDX spectra of the Ti-3864x alloys solution-treated at 800 ° C for 1 h before etching. No precipitates are observed in Ti-38640 alloy (Fig. 2a). However, white particles are clearly observed in Ti-38642, Ti-38644 and Ti-38646 alloys (Fig. 2b-d). Particles precipitate both on the grain boundaries and in the interior of β grains. The density of precipitated particles increases with the increase of Zr content. Figure 2e and f presents the EDX spectra of the matrix and particles of Ti-38644 alloy, respectively. The white precipitates are rich in Zr and Si. On the basis of this, the white particles may be Ti-Zr-Si silicides. Some previous literatures have reported that two types of hexagonal silicides precipitated in titanium alloys containing Zr and Si. The first is (TiZr)5Si3 (a = 0.780 nm, c = 0.544 nm), denoted as S1, and the second is (TiZr)6Si3 (a = 0.701 nm, c = 0.368 nm), denoted as S2 [14, 16].
 | Fig. 2 BSE images of Ti-38640 a, Ti-38642 b, Ti-38644 c and Ti-38646 d and EDX spectra of matrix e and white particles f in c |
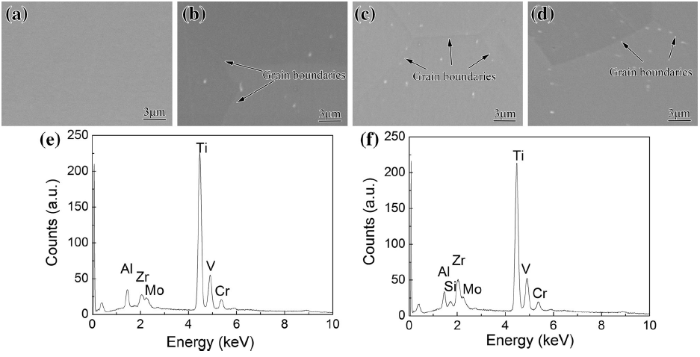
Figure 3 shows the TEM micrograph and the corresponding selected area electron diffraction (SAED) pattern of a precipitated particle in solution-treated Ti-38644 alloy. The precipitated particle is spherical in shape (Fig. 3a), identified as the (TiZr)6Si3 through the SAED pattern (Fig. 3b) and EDS information (Fig. 3c, d).
 | Fig. 3 TEM image a, corresponding SAED pattern b, energy-dispersive spectra of the matrix c and the precipitate d for the solution-treated Ti-38644 alloy |
In the present work, Si was introduced as an impurity, and the contents are the same in all these alloys. No silicides are observed in Ti-38640 alloy without Zr, whereas silicides precipitated in Ti-38642, Ti-38644 and Ti-38646 alloys containing Zr. This reveals that the added Zr promoted the formation of silicides. The formation mechanism may result from the two aspects. As mentioned previously, Zr has a strong surface activity [17, 18]. Therefore, it can be concluded that the addition of Zr is prone to induce the segregation of trace impurity Si. Simultaneously, the formation enthalpies of Zr-Si silicides are more negative than those of Ti-Si [19]. This indicates that Zr has a stronger affinity with Si than Ti.
Additionally, it was reasonable to assume that the grain refinement is due to the precipitated silicides hindering the movement of the grain boundaries. The higher Zr content induced more silicide precipitates, so that finer β grain size was obtained.
The tensile properties of Ti-3864x alloys are presented in Fig. 4. The yield strength (YS) and ultimate tensile strength (UTS) of the alloys are enhanced with increasing Zr content. The tensile elongation (El) and the reduction in area (RA) of Ti-38642, Ti-38644 and Ti-38646 alloys slightly decrease, as compared to Ti-38640. Nevertheless, the change in ductility is not apparent from Ti-38642 to Ti-38646. The higher ductility of Ti-38640 alloy originates from the single β phase microstructure, and dislocation motion is easier during tension. For Ti-38642, Ti-38644 and Ti-38646 alloys, the slightly lower ductility is related to the presence of silicides, which hinder the motion of dislocations. The pileup of dislocation around silicides may induce local stress concentration and crack initiation.
For Ti-38642, Ti-38644 and Ti-38646 alloys, the increase in strength is resulted from two strengthening mechanisms. Firstly, the fine dispersed silicides have an effect of precipitation strengthening. Secondly, grain refinement also contributes to the strength increase.
The fracture surfaces of Ti-3864x alloys are shown in Fig. 5. All of them are of dimple modes with shear lip zones. There are large amount of dimples spreading the major part of fracture surfaces. For Ti-38640 alloy, the fracture surface is rough, with large and deep dimples (Fig. 5a, e), indicating that the sample was experienced a large plastic deformation before fracture. This is consistent with its low yield strength and high ductility. With the increase of Zr content, dimples gradually become more uniform and smaller in size for Ti-38642, Ti-38644 and Ti-38646 alloys, as shown in Fig. 5f-h. This corresponds to the presence of silicide precipitates and the increase in quantity. The tensile fracture of Ti-3864x alloys is ductile.
1.No silicides are observed in Ti-38640 alloy. The formation of (TiZr)6Si3 silicides is resulted from the presence of trace impurity Si and the addition of Zr for Ti-38642, Ti-38644 and Ti-38646 alloys. The quantity of silicides gradually increases with the increase of Zr content.
2.The β grain size is refined significantly due to the presence of the fine dispersed silicides in Ti-3864x alloys.
3.The strength of Ti-3864x alloys increases with increasing Zr content. The increase in strength is attributed to the combination of precipitation strengthening and grain refinement. The slight ductility drop is related to silicide precipitates. The fracture mode of Ti-3864x alloys is ductile.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|