Hydroxyapatite fiber (HAF) was fabricated via a one-step hydrothermal route by using urea (CA), acetamide (AA) and propanamide (PA) as pH regulators, respectively, and the reaction time was only 0.5 h. The HAF synthesized by CA as the pH regulator was agglomerated with irregular morphology, while using AA or PA as the pH regulator, the HAF was composed of well-dispersed fibers with lengths larger than 200 μm. Two different morphologies form mainly due to the different hydrolysis rates of the pH regulators. Most of the fibers synthesized by the three pH regulators showed curving appearance, implying their outstanding flexibility. The technique in this paper allows the rapid formation of flexible HAF and may have wider applications in the restorations and reinforcement composites.
Bioceramics composed of calcium phosphates possess poor mechanical properties under compression, which prevents them from being used in load-bearing applications [1]. In comparison with common synthetic materials to reinforce calcium phosphate cements, hydroxyapatite (Ca10(PO4)6(OH)2, HA), which is one of the most widely used Ca-P based ceramics, exhibits advantages in chemical similarity to the mineral component of mammalian bones and teeth [2, 3, 4, 5]. However, the strength of HA needs to be improved for clinical practice. A fibrous version of HA, hydroxyapatite fiber (HAF), which has the potential to overcome the low mechanical properties with increased capillary-derived space and a large surface area, is a good choice [6]. In addition to its effects on bone growth, further applications in absorbing metal ions and organic pollutants could be realized by using the high aspect ratio HAF [7]. Also HAF could serve as a carrier for these ions in a similar fashion to a drug-delivery system [8].
Various methods for preparing HAF have been reported, including solid-state method [9], molten salt method [10], hydrothermal synthesis [11, 12, 13], homogenous precipitation method [14, 15], electrospinning technology [16, 17] and solvothermal treatment [18, 19]. Although the morphology has been improved to some extent, controllable preparation of both the phase and the morphology needs to be further studied.
To control the composition and morphology of the resulting crystals and to achieve large-scale uniform production, hydrothermal homogeneous precipitation is a relatively easy method, showing a slow reaction rate by combining the best characteristics of the hydrothermal synthesis and the homogenous precipitation method. Recently, urea (CA), acetamide (AA) and propanamide (PA) have commonly been used as pH adjusting agents to prepare HAF hydrothermally [20, 21]. Although some progress has been made, most of the obtained products exhibit a short and straight whisker-like morphology, which appears to be brittle. Also little about the difference among three pH regulators has been previously reported. Furthermore, the process is highly time-consuming and usually takes over 10 h [20, 21].
Herein, we report a one-step synthesis of HAF by a hydrothermal homogeneous precipitation method in only 0.5 h without the addition of any structure-directing agents and templates. CA, AA and PA are used to control the pH of the solution, respectively. Most of the as-prepared fibers are ultralong with lengths larger than 200 μ m and curving appearance, which imply good flexibility.
Calciumnitrate tetrahydrate [Ca(NO3)2· 4H2O], diammonium hydrogen phosphate [(NH4)2HPO4], urea [CO(NH2)2], acetamide [CH3CONH2] and propionamide [C3H7NO] were supplied by Sinopharm Chemical Reagent Corporation (Shanghai, China). All chemicals used for the synthesis were of analytical grade and were used as received without any further purification.
In a typical procedure, 100 mmol/L Ca(NO3)2· 4H2O solution and 60 mmol/L (NH4)2HPO4 solution were mixed with a Ca/P molar ratio of 1.67. Fifty milliliters of aqueous solutions of CA, AA and PA at 1 mol/L was added into 50 mL calcium and 50 mL phosphate to control the pH of the synthesis solution, respectively. All of the initial pH values of the mixed reactions were adjusted to 3.0 by 0.05 mol/L HNO3 solution. After that, the obtained solutions were transferred into Teflon-lined stainless steel autoclaves and treated hydrothermally at 180 ° C for 0.5 h. Then, the resulting suspensions were collected and washed thoroughly with deionized water and anhydrous ethanol three times and finally dried at 80 ° C for 4 h in air.
The crystalline phases of the products were identified using an X-ray diffractometer (XRD, Rigaku D/max-γ B 40 kV, CuKα radiation, λ = 0.15418 nm). The morphology and selected area electron diffraction (SAED) of the as-synthesized HA structures were characterized by field emission scanning electron microscopy (FE-SEM, SU-70, 15 kV) and high-resolution transmission electron microscopy (HRTEM, JEM-2100, 200 kV). For FE-SEM analysis, the samples were sputter-coated with gold to increase the conductivity.
Figure 1 displays the XRD patterns of the products obtained at 180 ° C for 0.5 h by three different pH regulators and the line pattern of standard HA (JCPDS No. 09-0432). All the main peaks of the products can be indexed into HA, indicating that the main phase of the obtained products is all HA. The diffraction pattern of the product when CA is used as the pH regulator (Fig. 2a) is in accordance with standard HA, and its crystallinity is lower than the other two. For the products synthesized by AA or PA regulator (Fig. 2b, c), the diffraction intensities of (100), (200) and (300) reflections, which corresponds to the a-plane of HA crystals, are more intense than the one in Fig. 2a. Meanwhile, the shift of the most intense peak from (211) to (300) and loss of peak (112) are found when AA or PA is used as the pH regulator, suggesting preferential orientation growth of HA [22], which is consistent with other literatures [20, 21].
 | Fig. 1 XRD patterns of the as-synthesized products obtained at 180 ° C for 0.5 h by different pH regulators: a urea, b acetamide, c propanamide |
Figure 2 shows the FE-SEM images of the products obtained at 180 ° C for 0.5 h by different pH regulators. Compared to those formed with AA and PA, the product contains flower-like aggregates with irregular morphology by CA regulator. These aggregates are where HAF nucleates and grows (Fig. 2a, b). Most of the fibers with lengths larger than 200 μ m show flexibility, implying the deformed appearance without fracturing (Fig. 2a, c, e). Different from the HAF fabricated by electrospinning [16, 17], the fibers here are clean with a smooth surface.
In order to show the flexible fibers more clearly, we focus on the typical morphology of the three obtained products (Fig. 3). The as-prepared ultralong HAF is highly flexible and can be bent and even rolled without being broken. Mechanical approach to characterize the flexibility of HAF has not been found yet, and the way to describe the flexible behavior of fibers is mainly through their bent morphology [7, 18, 19].
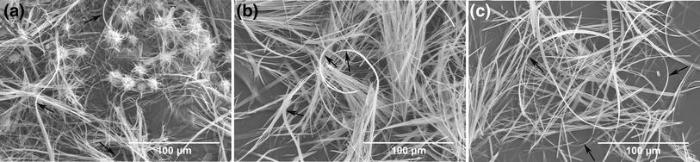 | Fig. 3 Typical FE-SEM images of the flexible fibers obtained at 180 ° C for 0.5 h by different pH regulators: a urea, b acetamide, c propanamide |
The crystalline structure of HAF prepared at 180 ° C for 0.5 h by CA is further analyzed by HRTEM as shown in Fig. 4. The prepared HA contains ribbon-like structures (Fig. 4a), which are in accordance with the FE-SEM images (Fig. 2a). Spots in the SAED pattern of the ribbon near the (000) plane (as shown in Fig. 4b inset) are identified as (002), (110) and (112) which match the vector relationship of the crystal planes well and are taken from [\(\bar{2}\, 20\)] zone axis. Figure 4c demonstrates the one-dimensional lattice fringes along with the interplanar spacing of 0.3414 nm, which can be indexed to the (002) plane of HA crystal, in agreement with the XRD results, suggesting that the ribbons are elongated along c-axis ([001] direction). Similar observations have been made for HAF obtained under hydrothermal conditions by other groups [20, 22].
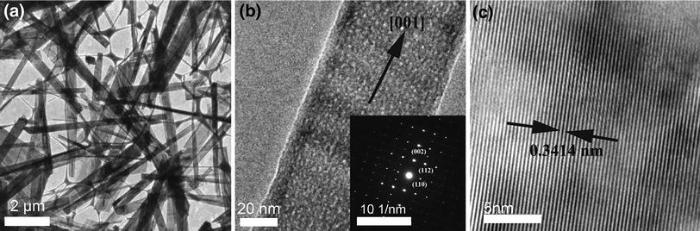 | Fig. 4 TEM images of the HAF obtained at 180 ° C for 0.5 h by urea: a TEM with low magnification, b TEM and SAED pattern (inset), c HRTEM |
From the above results, it can be seen that the pH regulators mainly influence the morphology of the HAF via a hydrothermal route. The pH values of the solutions after the reaction are 7.6, 3.7 and 3.8 for CA, AA and PA, respectively. Compared with AA and PA, CA shows a quicker hydrolysis rate. The pH rises rapidly, giving larger numbers of nuclei, and thus aggregates form. Different hydrolysis rates result in the HAF having different morphologies.
Mineral precipitation usually occurs in a supersaturated solution. Referring to the supersaturation of HA, S can be defined by Eq. (1) [23],
$$s = \left( {\frac{{\alpha_{{{\text{Ca}}^{2 + } }}^{10} \alpha_{{{\text{PO}}_{4}^{3 - } }}^{6} \alpha_{{{\text{OH}}^{ - } }}^{2} }}{{K_{\text{sp}} }}} \right)^{1/18}\ \ (1)$$
where α is the ion activity and Ksp is the solubility of HA product, which is a constant at a specific temperature. The initial pH is 3.0 and cannot be undersaturated with HA. However, the nucleation of HA can be triggered by OH- (Eq. 1) or pH. In the computer simulation and experiment, it is noted that nucleation occurs at nanoseconds [24], much faster than the completion of the OH- diffusion process in the solution. Compared with conventional NH3· H2O adjusted dropwise, which results in the heterogeneous solution condition [25], the uneven distribution of OH- can be greatly reduced by using CA, AA and PA as the pH regulator. The reaction is described by Eqs. (2-4).
$${\text{CO}}\left( {{\text{NH}}_{2} } \right)_{2} + 3{\text{H}}_{2} {\text{O}} \to 2{\text{NH}}_{4}^{ + } + \, 2{\text{OH}}^{ - } + {\text{ CO}}_{2} .\ \ (2) \\ {\text{CH}}_{3} {\text{CONH}}_{2} + 2{\text{H}}_{2} {\text{O}} \to {\text{NH}}_{4}^{ + } + {\text{ OH}}^{ - } + {\text{CH}}_{3} {\text{COOH}} .\ \ (3) \\ {\text{CH}}_{3} {\text{CH}}_{2} {\text{CONH}}_{2} + 2{\text{H}}_{2} {\text{O}} \to {\text{NH}}_{4}^{ + } + {\text{OH}}^{ - } + {\text{CH}}_{3} {\text{CH}}_{2} {\text{COOH}} .\ \ (4)$$
The release of NH4+ by heating can result in the relatively homogeneous pH field and provide the “ instant” OH- for HAF formation. At high driving force, the interface could produce a continuous growth to allow the formation of ultralong HAF [21].
We expect that the method could be scaled-up for high-yield production of HAF, and that the prepared HAF is promising for applications in various fields such as bone tissue restoration and composite materials.
Flexible HAF with lengths larger than 200 μ m was synthesized in only 0.5 h by one-step hydrothermal homogeneous precipitation method without any structure-directing agents and templates. The HAF synthesized by CA as the pH regulator is agglomerated with irregular morphology, and the preferred orientation of the crystal is the c-axis direction. While using AA or PA as the pH regulator, the HAF is composed of well-dispersed fibers with a uniform morphology. Two different morphologies form due to the different hydrolysis rates of the pH regulators. This method is simple, surfactant-free and environmentally friendly, which may have wider applications in HA-based bioceramics.
Acknowledgments:This work was financially supported by the Fundamental Research Funds of Shandong University (No. 2015JC018), the Jiangsu Province Science Foundation for Youths (No. BK20140412) and the Shandong Province Young and Middle-Aged Scientists Research Awards Fund (No. BS2013CL030).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|



