Metal oxides can be used as a series of new and effective anti-bacterial agents. In this study, four concentrations of ZnO nanoparticles (0.2, 0.5, 0.7 and 1.0 mol/L) were synthesized using a low-temperature sol-gel method annealed at 400 and 550 °C. The products were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR). XRD results show the hexagonal wurtzite structure of the nanoparticles with the grain size in the range of 38-43 nm. TEM micrographs exhibit a polyhedral form of the synthesized nanoparticles. The antimicrobial activity of different concentrations of nanoparticles against Salmonella typhi PTCC 1609 was determined by disk diffusion and agar dilution method at five concentrations of 10, 5, 2.5, 1.25 and 0.625 mg/mL. Analysis shows that the prepared ZnO nanoparticles have a very effective antimicrobial activity against Salmonella typhi. This activity increases by reducing the size of nanoparticles and increasing their content in the bacterial growth medium.
Nowadays multidrug resistance infections have become a challenge for the medical community. Treatment of these microorganisms is a challenge because resistance limits dramatically therapeutic options. So the discovery of new, safe and natural antimicrobial agents to combat these pathogens is essential [1]. One of the most efficient metal oxide semiconductors is ZnO that has a binding energy of 60 meV with a wide energy gap of 3.37 eV [2, 3, 4, 5, 6]. Because of its unique electrical, optical, catalytic, physical and chemical properties, ZnO is increasingly used for various applications in recent years [7, 8, 9, 10, 11]. ZnO nanoparticles (NPs) are supposed to be biodegradable and nontoxic for the nature. Meanwhile, eukaryotic cells are resistant to low concentrations of them [12]. Therefore, the particles of ZnO with one dimension in the range of 1-100 nm resulting in extensive reactions with the bacterial surface [13] can be used as an efficient bactericidal agent against both Gram-positive and Gram-negative pathogens like Escherichia coli O157:H7, Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis and Pseudomonas fluorescens [14, 15, 16]. The reports by Azam et al. [17] and Tayel et al. [18] reveal that the Gram- positive strains are more susceptible to these NPs that related to the construction and thickness of the membrane and wall of bacterial cells.
According to the previous studies, effective bactericidal activity of ZnO NPs is related to their size and concentration [19, 20, 21]. The study by Xie et al. [15] showed that ZnO NPs have a strong antibacterial activity against C. jejuni that creates changes in morphology of this bacterium by increasing oxidative stress gene expression and membrane leakage, even at low concentrations. In comparison with the ordinary ZnO powder, nanostructured ZnO shows more effective antibacterial action [22]. Raghupathi et al. [12] reported that ZnO NPs with smaller size have higher antibacterial activity than larger size NPs on both Gram-positive and Gram-negative bacteria. However, so far, they haven’ t shown antibacterial activity against high-temperature and high-pressure resistant spores, and the factors related to the crystal structure of nanoparticles are not very effective on their antibacterial activities [23].
Several methods have been used to synthesize ZnO nanoparticles such as hydrothermal, solvothermal, microwave irradiation and sol-gel [6, 12, 19, 21]. Among these methods currently used to produce ZnO NPs, the sol-gel remains very useful economic route [24].
In the sol-gel process, an oxide network is obtained via inorganic polymerization reactions in solution. An idea to use polybasic hydroxy carboxylic acids in syntheses of oxides goes back to Pechini [24]. The main goal of this study is to prepare ZnO NPs via the quite facile but efficient sol-gel Pechini method and evaluate the pertinent antibacterial activity against Salmonella typhi. In the meantime, the effective agents of ZnO NPs against Salmonella typhi will be investigated.
There are some reports on the antibacterial activity of ZnO NPs against Salmonella typhi [6, 18]. To the best of our knowledge, reports on the antibacterial activity of sol-gel Pechini derived ZnO NPs against Salmonella typhi are very rare. By post-heat treatment procedure, sol-gel enables us to tune the size of nanoparticles. In the present study, two different annealing temperatures are tested to investigate the effect of the particle size on the antibacterial performance of ZnO nanoparicles.
Species of Salmonella causes food-borne illnesses [18]. Salmonella typhi is a host private pathogen that causes typhoid or enteric fever. It is a multidrug-resistant bacterium, and the vaccines to establish a lasting protective effect were not successful [25]. Hence, development of new antibacterial agents like ZnO NPs seems to be so necessary that we are chasing in this work.
Zinc oxide nanoparticles were synthesized by the low-temperature sol-gel Pechini method. All chemicals were provided by Merck. The chemicals used for synthesis were zinc acetate dihydrate, methanol and diethanolamine. Muller Hinton Broth (MHB), blood agar (BA) and Bismuth sulfite agar (BSA) were used as the growth medium for bacteria, and Trypticase Soy Agar (TSA) were applied for anti-bacterial tests.
To prepare 0.2, 0.5, 0.7 and 1.0 mol/L solutions, different amounts of zinc acetate (1.31, 3.29, 4.6 and 6.58 g) were weighed and mixed well in 20 mL methanol using a magnetic stirrer for 0.5 h. In another beaker, 0.7, 1.5, 2.2 and 3 mL of diethanolamine were blended well in 20 mL methanol and stirred for 0.25 h. The methanol solution was then mixed with zinc acetate solution under continuous stirring for 1 h. Hydrolysis process occurred when the resultant transparent sol was left for 24 h at room temperature. The prepared solutions were then preheated at 180 ° C for 2 h. Subsequently, the produced yellow gels were annealed in a precalibrated electric furnace at two different temperatures of 400 and 550 ° C for 2 h. Eventually, ZnO NPs were obtained in white powder form at different concentrations.
The structure of the samples was studied by X-ray diffraction (XRD) method using a XRD 6000, Shimadzu system with CuKα radiation (λ = 0.15406 nm). The dry diameter and size distribution of the various NPs were measured by a transmission electron microscope (TEM) (CM120, Philips, the Netherlands). Fourier transform infrared spectroscopy (FTIR) absorption was measured for KBr-supported samples over the frequency range of 4000-400 cm-1 and at a resolution of 4 cm-1, using a model SHIMADZU, FTIR-8400S.
Standard bacterial cultures Salmonella typhi PTCC 1609 were procured from the Persian Type Culture Collection. The bacterial cultures were streaked to check the purity, and a single colony was inoculated in selective medium of BSA. Blood agar was used in growing and maintaining the bacterial cultures. Then the pure cultures of S. typhi were subcultured aerobically in MHB at 37 ° C on a rotary shaker at 150 rpm for 24 h before using as target organisms. The density of bacterial isolates was adjusted to an optimal density of 0.5 McFarland standards. A stock suspension was prepared by suspending of synthesized NPs (8 stokes) in sterile Muller Hinton Broth to yield the final concentration of 0.01 mg/mL followed by constant stirring to obtain a uniform colloidal suspension. Further dilutions were prepared at the required concentrations of 10, 000, 5500, 2550, 1250 and 625 µ g/mL, respectively.
The antibacterial activity of ZnO nanoparticles against S. typhi PTCC 1609 was tested using modified Kirby-Bauer diffusion method [26] and MIC and MBC determination by broth dilution tests [27]. Paper disks of 5 mm diameter were cut and sterilized. After inoculation and cultivation of target bacteria on the top of TSA, disks were placed in the selected area on different plates. Using a micropipette, 10 µ L of NPs suspensions was poured onto the surface of each disk. On all plates, after overnight incubation at 37 ° C, different levels of zone of inhibition were measured. Each experiment was done in triplicate.
Determination of the minimum inhibitory concentration (MIC) by the microdilution method in culture broth is a way to evaluate the antibacterial activity of the produced NPs. Five dilutions were prepared for each sample. Each set was inoculated aseptically with 100 µ L of target bacterial suspension (0.5 McFarland standards). Nanoparticle-free medium and bacteria-free medium were used as the control positive and the control negative, respectively. The inoculated sets were incubated at 37 ° C for 24 h. MIC was defined as the lowest ZnO concentration that completely inhibited bacterial growth at the end of 24 h of incubation.
The MBC was determined by subculturing of 0.1 mL of the medium drawn from the culture tubes after 24 h on BSA. The growth was scored for relative numbers of the bacterial colonies. The lowest concentration of the antimicrobial agent causing negative growth (fewer than three colonies) was considered as the MBC. Viable bacterial colonies were counted and recorded by the naked eye.
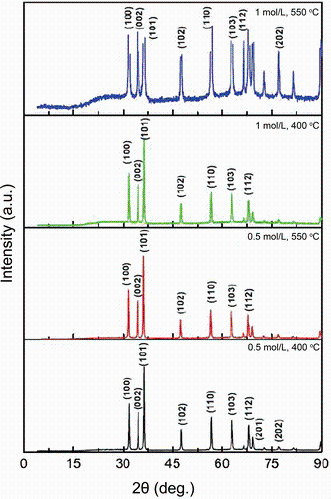
The XRD patterns of ZnO NPs are shown in Fig. 1. The diffraction patterns can be well indexed to be a hexagonal wurtzite structure of ZnO (JCPDS No. 36-1451). Additionally, no impurity peaks were observed in the patterns, and the prepared zinc oxide NPs showed a single phase in the sample. With increasing the annealing temperature, the peaks position did not change, but their intensity increased. This peak intensity enhancement reflects the enhancement of crystallinity and overall the quality improvement of the nanoparticle crystalline structure.
XRD pattern was used to evaluate the size of ZnO NPs by the well-known Scherrer’ s formula [28]
$$\it {\text{D}} = \frac{{0.9 {{\lambda}}}}{{{{\beta cos\theta}}}} . \ \ (1)$$
where λ is the wavelength of X-ray radiation and β is the full width at half maximum (FWHM) of the peaks at the diffracting angle θ . Different temperatures and molarities led to the production of different sizes of ZnO NPs. The average size of the nanoparticles varied from 38.07 to 43.69 nm. With increasing the reaction temperature, gathering of small grains occurs, so large grains were obtained and the crystallinity was improved [5, 29, 30]. When the particle size reduces, the surface-to-volume ratio and so the surface atoms in the NPs increase. This can improve the surface reaction of the nanoparticles [31].
The size and morphology of the prepared ZnO NPs were analyzed by TEM and are presented in Fig. 2. The micrographs reveal that the products consist of polyhedral particles with the average size of 30-40 nm which is in good agreement with XRD results. However, the lowest value of the nanoparticle size (i.e., 30 nm) differs about 27% from the corresponding XRD value. The average grain sizes calculated from XRD patterns are related to different growth orientations. Hence, such a difference is expected.
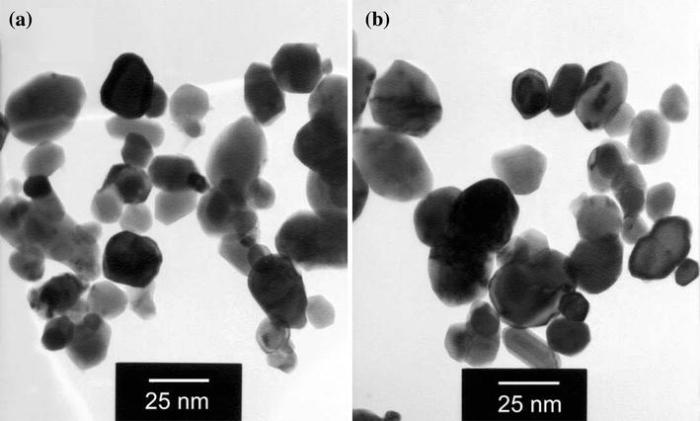 | Fig. 2 Representative TEM images of ZnO NPs prepared in 1 mol/L concentration annealed at 400 ° C a, 550 ° C b |
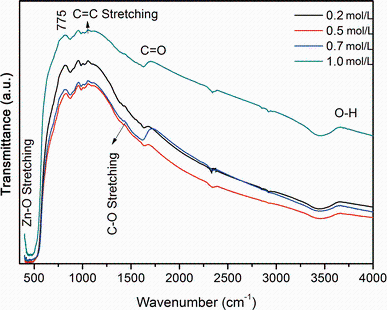
The functional groups of ZnO NPs (0.2, 0.5, 0.7 and 1.0 mol/L) were analyzed using FTIR spectrometry and are shown in Fig. 3. The FTIR peaks obtained for ZnO nanoparticles were in close resemblance with the peaks reported by Akbar et al. [6]. FTIR spectra of zinc oxide NPs exhibited vibration in the region around 550 cm-1, which can be attributed to the vibration of Zn-O and confirming the formation of ZnO NPs [6]. Several peaks in the range of 900-3853 cm-1 were found due to the presence of different functional groups of other organic materials used in the preparation of the nanoparticles. For instance, the peak at 1000 cm- 1 shows the C=C stretching vibration. The peaks at 1250 cm- 1 and 1750 cm- 1 represent the C-O and C=O stretch, respectively. The absorption peak at 3550 cm-1 indicates the presence of hydrogen-bonded O-H stretch [11].
The antibacterial effects of ZnO NPs against S. typhi were studied by determining the zone of inhibition (Fig. 4). The statistical results represented in Fig. 5 show that the best zone of inhibition against S. typhi with diameter of 3.1 cm refers to 1.0 mol/L synthesized NPs annealed at 400 ° C in 10 mg/mL of suspension. Results of the previous work on the antibacterial activity of ZnO NPs prepared by different methods against both Gram-negative and Gram-positive bacterial strains are given in Table 1 for comparison.
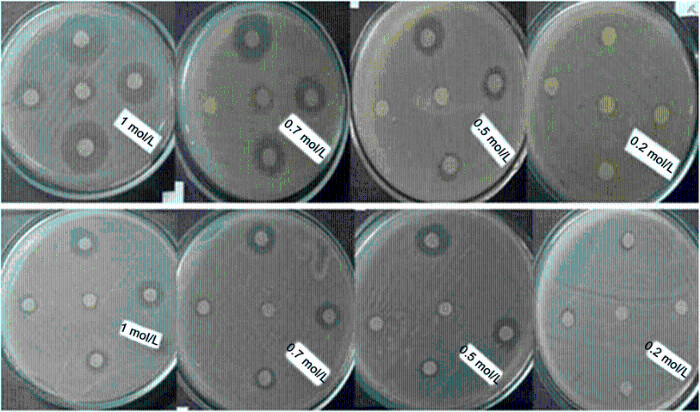 | Fig. 4 Antibacterial activity of ZnO NPs against S. typhi. Upper: synthesized at 400 ° C and below: synthesized at 550 ° C in four molarities |
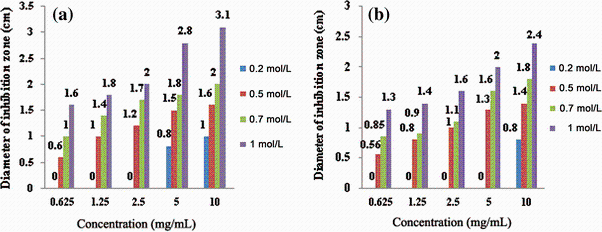 | Fig. 5 Zones of inhibition produced by different concentrations of zinc oxide NPs synthesized at 400 ° C a, 550 ° C b |
| Table 1 Comparative antibacterial activities of undoped and doped ZnO nanoparticles prepared by different methods against pathogenic bacterial strains |
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for different molarities of the nanoparticles synthesized at 400 and 550 ° C are presented in Table 2. Number of colony-forming unit (CFU) of S. typhi after an overnight incubation at the presence of different concentrations of ZnO NPs are shown in Fig. 6. In the control plates (NPs-free), CFU was 288. It is observed that with high loading of ZnO NPs, the numbers of CFU reduce. One of the most effective agents on the antibacterial activity of zinc oxide NPs is their size and surface charge [20, 31, 32]. For example, the typical size of the nanoparticles is almost 250 times smaller than that of a typical bacterial cell. Therefore, they can penetrate deeply enough the bacterial membrane and affect it efficiently [33]. Raghupathi et al. [12] concluded that among MgO, TiO2, CuO, CaO, CeO2 and ZnO, the nanoparticles of ZnO have a size-dependent effect so that with decreasing the particle size, bacterial cells decreased too. When the size of NPs reduces, the ratio of the surface area to volume increases and the number of reaction sites and oxyradicals generation increases too, which in turn leads to more interaction with the cells and improves the permeability of bacteria for entering the NPs [34, 35]. That is because in this case, for encompassing a bacterial colony, a great number of the particles are needed. So, releasing of active oxygen species on the surface of the colony increases, and antibacterial activity occurs with more power. It means that the reactive oxygen species generated from the surface would be increased which corresponds to an increase in the number of ZnO particles per unit volume. Indeed, the smaller size of ZnO NPs would have more power for inhibiting the growth of the bacteria due to increment of reactive oxygen species production and the NPs accumulation [14, 18, 36]. On the other hand, when the particle size decreases, the amount of dissolved Zn2+ ions in the nanoparticle included suspension increases and the pertinent pH changes. Both of these factors cause reduction of bacterial activity [12, 37]. As it comes from Fig. 4, in the same molarity, the size of NPs is very effective on their antibacterial activity. Thus, with reduction of NPs size in the same molarity, their antibacterial activity increases. The maximum diameter of the inhibition zone for 1 mol/L NPs at 400 and 550 ° C was 3.1 and 2.4 cm, respectively. However, it seems that the molarity of the solutions is more effective in this case. It means that lower molarity of NPs synthesized at 400 ° C with smaller size has lesser antibacterial effect than the NPs synthesized at 550 ° C with bigger size. At the same time, inhibition zone of NPs synthesized at the same temperature in higher molarity was wider. As it is shown in Fig. 6, same results were obtained from the colony counting method. From this figure, NPs synthesized at 400 ° C related to the solution of 1.0 mol/L in concentration of 10 mg/mL were more effective in the reduction of CFU than the NPs synthesized at 550 ° C with the same molarity. Also, NPs prepared from the solutions with higher molarities were more effective in reduction of CFU of S. typhi.
| Table 2 Determination of MIC and MBC for different molarities of nanoparticles synthesized at 400 and 550 ° C |
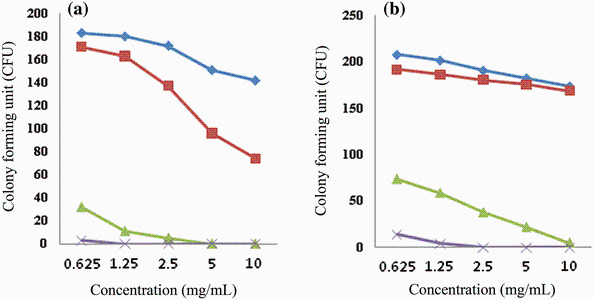 | Fig. 6 Number of colony-forming units (CFU) of S. typhi after overnight incubation at the presence of different concentrations of ZnO NPs synthesized at 400 ° C a, 550 ° C b in four molarities |
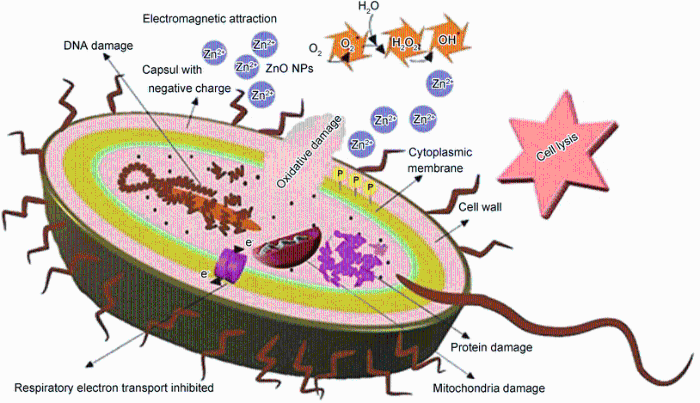
Actually, an electromagnetic attraction occurs between the bacteria (with negative charge) and ZnO NPs (with positive charge) that leads to binding between them [38]. Indeed, the ZnO NPs interact with the membrane lipids and thiol groups (-SH) of enzyme and proteins that are important for the bacterial respiration and the transport of important substance across the cell membrane and within the cell [10, 39]. Furthermore, ZnO NPs can penetrate inside the bacterial cell and inactivate the phosphorus- and sulfur-containing compounds such as DNA and their enzymes [23, 37]. Production of high levels of reactive oxygen species (ROS) such as hydrogen peroxide impacts an important role in this function. Actually, damaging of membrane, DNA and cell proteins, is as a result of ROS generation that leads to the prevention of bacterial growth and death [12, 17, 39, 40, 41]. This process has been shown in Fig. 7 schematically.
During the present study, different concentrations with different molarities of zinc oxide NPs were tested and the best concentration that can have the most effective antibacterial property against S. typhi was found. Wang et al. [20] reported that ZnO NPs have strong antibacterial activity against E. coli K88 and the activity increases as the concentration of zinc oxide NPs increases. This result was achieved by increasing the concentration of ZnO NPs in wells and disks [42]. High concentrations of ZnO NPs produce enough hydrogen peroxide and damage the bacterial membrane that causes DNA decomposition and activates caspase protein [43]. Sequential activation of caspase protein plays a central role in the execution phase of cell death. Also, dehydrogenase enzyme activity is reduced due to the increased encounter between oxygen and this enzyme [44]. A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by a reduction reaction that removes one or more hydrogens from a substrate to an electron acceptor. On the other hand, the increase of ZnO NPs concentrations leads to mitochondrial function disorder and leakage of lactate dehydrogenase [18]. So, inhibition of bacterial growth is completely related to the concentrations of ZnO NPs and the initial number of bacterial cells. In the present study, in high concentrations of NPs, the number of dead cells increased (Fig. 6). It means that inhibition zones increased and CFU decreased. In higher molarities of NPs, no bacterial growth was observed and inhibition zones with excess diameters were obtained. As a matter of fact, antibacterial activity of ZnO NPs is a dose-dependent issue. However, it cannot be claimed for sure that the antibacterial activity of NPs is proportional to the concentration of NPs. It may be due to occurrence of resistance in bacterial strains in higher concentrations. Meanwhile, it is seen from Table 2 that MIC and MBC values of 1.0 mol/L ZnO NPs synthesized at 400 ° C are equal. It means that in this concentration, NPs have bactericidal effect. As a result, the larger surface area (lower grain size), higher concentration and molarity of NPs caused higher antibacterial activity of ZnO nanoparticles.
The antimicrobial activity of ZnO NPs against Salmonella typhi was investigated in this study. ZnO NPs were synthesized via a facile modified sol-gel process and characterized by XRD, TEM and FTIR. XRD patterns indicated that the pure wurtzite structure of ZnO NPs was formed. It was observed that the particle size increased as the annealing temperature increased. TEM images revealed that the products consist of polyhedral particles with the average size of 30-40 nm. Results of two applied antimicrobial methods showed that the synthesized ZnO NPs have an effective antibacterial activity against S. typhi. This activity increased by reducing/increasing the size/molarity of NPs and also increasing their content in the bacterial growth medium. It was concluded from the study that, use of ZnO nanoparticles is an important strategy to control the food-borne pathogens, particularly S. typhi. Since the use of ZnO nanoparticles is acceptable in food industry, its use can be helpful in different ways, because such a material is not only antibacterial but also a source of zinc as a food supplement.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|