Magnesium oxide (MgO) is one of the metal oxides having unique properties with numerous potential industrial applications. In this study, MgO and vanadium-doped MgO nanoparticles were synthesized by sol-gel method in 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4] and 1-octyl-3-methylimidazolium tetrafluoroborate [OMIM][BF4] ionic liquids. Vanadium-doped MgO nanoparticles exhibited nanosphere and nanorod morphologies with 40-80 nm in particle size, primarily due to the influence of ionic liquids as demonstrated by high-resolution scanning electron microscopy and transmission electron microscopy. Characteristics of nanoparticles were also studied by thermal gravimetric analysis, X-ray diffraction and energy-dispersive X-ray spectroscopy. Photodegradation ability of synthesized nanoparticles was evaluated for methylene blue (MB) in specially designed UV reactor. Photodegradation is found to be dependent on doping, and particle characteristics change due to the influence of ionic liquid. The ionic liquid-assisted vanadium-doped MgO nanoparticles showed good reusability under UV irradiation and MB degradation ability under visible light.
Magnesium oxide (MgO), one of the important alkaline earth oxides, has been extensively used in catalysis, antibacterial materials, refractories, paints and superconductors due to its unique optical, electronic, thermal and chemical properties [1]. It is also used for organic transformation reactions as catalyst, modifier, promoter and support. There are several routes for synthesizing magnesium oxide such as thermal decomposition of magnesium hydroxide, magnesium carbonate or magnesium nitrate as precursor. Type of precursor as well as synthesis conditions affects its properties such as the particle morphology, the size and the surface area [2]. Among different types of magnesium oxides, nanosized magnesium oxide is preferred owing to better physical properties as well as enhancement in the application performance. One of the interesting applied areas explored for magnesium oxide is photocatalysis where it is used to support metals to tackle organic pollutants. Improved photocatalytic activity has been observed by modifying surface properties of magnesium oxide by doping, co-precipitation of metal, chelation and mixing of two photoconductors [3].
Several studies have been reported on doping of transition metals on magnesium oxide. Kantam et al. [4] synthesized ruthenium nanoparticles stabilized on the nanocrystalline magnesium oxide by the incorporation of choline hydroxide for transfer of hydrogenation of various carbonyl compounds with excellent yields. Layek et al. [5] developed a simple and elegant synthesis method for gold nanoparticles via counter ion stabilization of AuCl4 on nanocrystalline magnesium oxide support followed by sodium borohydride reduction. Super magnetic nanoparticles consisting of magnesium, manganese and iron were synthesized by sol-gel process using diethylene glycol (DEG) as reducing agent as well as surface stabilizer exhibiting very good stability in water due to DEG coating on the surface [6].
Vanadium, one of the transition metal elements, contains partially filled d-orbital electrons and has similar electronic properties like titanium of titanium dioxide synthesized by several methods such as sol-gel and hydrothermal methods [7]. Vanadium pentoxide or oxide is also found to be applied to the microelectronics, solid-state ionics, sensing, energy storage devices, catalysis, biosensors adsorption, photocatalysis, etc. Synthesis of vanadium-containing magnesium oxide by different methods has been widely reported. Vanadium carbide (VC) was prepared via a simple and novel route by the reaction of metallic magnesium powder with vanadium pentoxide (V2O5), citric acid (C6H8O7) at 650 ° C or potassium acetate (CH3COOK) at 500 ° C in an autoclave [8]. Magnesium-vanadium nanoparticles were prepared by hydrogen plasma-metal reaction (HPMR) method [9]. Novel visible light composite photocatalyst Ag/AgCl/BiMg2VO6 was synthesized through a precipitation-photoreduction method [10].
Mg-doped VO2 nanoparticles were successfully prepared via hydrothermal synthesis. When the dopant quantity increased to 3.8 at.%, the properties of the films were excellent for the application of VO2 in smart windows among others [11]. Magnesium oxide was found to show activity for the reduction of carbon dioxide to carbon monoxide under photoirradiation using hydrogen as a reductant [12]. Uda et al. [13] reported that hydrogen gas can be easily produced from water at room temperature using Mg nanopowder (30-1000 nm particles, average diameter 265 nm). The Mg(OH)2 flakes were formed on the surface of the Mg particles as a result of this reaction [10]. The magnesium-vanadium oxide composite nanoparticles were synthesized by a solution containing a magnesium salt and a vanadium salt using an organic polymer to manufacture magnesium-vanadium oxide composite nanoparticles [14].
Using ionic liquids in nanoparticle by sol-gel method leads to the improvement of properties. An ionic liquid is defined as organic salt, consisting of bulky anion and cation connected by electrostatic and H-bonded network. Surfactants, such as Tween-80 and/or polyethylene glycol (PEG), are required if nanoparticles need to be stabilized in aqueous media for synthesis of nanoparticles such as silver nanoparticles [15]. Ionic liquid eliminates the use of such surfactants if added during sol-gel process.
Several researchers demonstrated magnesium-containing nanoparticles synthesis using ionic liquid. Highly crystalline magnesium hydroxide was obtained by sol-gel process using magnesium nitrate with ammonium hydroxide in a hydrophilic ionic liquid, 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), as a medium [16]. Nanocrystalline MgO nanoparticles in cubic phase structure were synthesized via sol-gel process using 1-n-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. This route shows a new window to improve the physico-chemical properties of MgO nanoparticles [17]. Pyran derivatives and coumarin derivatives were synthesized using zinc oxide/magnesium oxide (ZnO/MgO) in an ionic liquid (i.e., [BMIM]BF4) reaction medium [18]. The MgO nanoparticles were synthesized by conventional process and the ionic liquid, 1-n-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4], assisted process to produce controlled size MgO nanoparticles with different morphologies [19].
Use of ionic liquid for synthesis of magnesium oxide and doping with vanadium pentoxide by sol-gel method has not been much explored specially for photocatalysis applications. In the present work, we synthesized magnesium oxide/vanadium-doped magnesium oxide nanoparticles using [BMIM][BF4] and [OMIM][BF4] ionic liquids (Fig. 1) with an objective to understand the effect on nanoparticles properties and photocatalytic activity. Synthesized nanoparticles were characterized by thermal gravimetric analysis (TGA), transmission electron microscopy (TEM), high-resolution scanning electron microscope (HR-SEM), X-ray diffraction (XRD) and evaluated for photocatalytic activity of methylene blue (MB) degradation in specially designed UV reactor. Synthesized nanoparticles were further explored for MB degradation under visible light irradiation and its reusability potential under UV light irradiation.
A 98% VO(OiPr)3 (vanadium oxytriisopropoxide), 99% isopropanol, 98% 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4], 1-octyl-3-methylimidazolium tetrafluoroborate [OMIM][BF4] were purchased from Sigma-Aldrich. Demineralized water is used for all hydrolysis experiments. A 20% commercial ammonia in water and laboratory-reagent-grade acetonitrile (Sigma-Aldrich) were used for neutralization and washing of reaction products. Commercially available methylene blue was purchased from S. D. Fine Chemicals, Mumbai, India.
Calculated amount of caustic flakes diluted in deionized water was taken in 500-mL round-bottom flask. It was kept in an oil bath for control of reaction temperature. In the case of MGBM and MGOM (refer Table 1 for abbreviations), ionic liquid was also added to ensure the presence during hydrolysis. Quantity of ionic liquid was kept 1.5 times of magnesium dichloride precursor mole. Magnesium dichloride solid was added very slowly (in 10 min) to caustic solution. The solution was mixed using magnetic spin bar at 1000 rpm. Reaction mass was heated to 70 ° C and hold for 2 h to ensure complete hydrolysis. Formation of white/milky solid was observed immediately on magnesium dichloride addition. As the reaction proceeded, solution became thick due to the formation of magnesium hydroxide. After 2 h of reaction, solution was cooled to room temperature and kept overnight for 16 h without stirring to complete gelation/condensation process. Magnesium hydroxide particles were washed three times with deionized water and separated from solution using centrifuge at 4000 rpm for 5 min. In the case of MGBM and MGOM, acetonitrile was used to remove/extract ionic liquid for washing of magnesium hydroxide nanoparticles followed by centrifuge separation. The nanoparticles thus obtained were kept at 110 ° C for 2 h to evaporate free water as well as acetonitrile. Later, magnesium hydroxide nanoparticles were heated at 400 ° C for 4 h in a muffle furnace to remove excess solvents (deionized water, acetonitrile) and ionic liquid. Calcination converts magnesium hydroxide nanoparticles to magnesium oxide nanoparticles by the removal of water molecules. All calcined magnesium hydroxide nanoparticles are referred as magnesium oxide in further discussion. Reactant quantities for experiments are taken as listed in Table 2.
| Table 1 Samples description |
| Table 2 Reactant quantity for synthesis of magnesium hydroxide and vanadium-doped magnesium hydroxide nanoparticles |
Magnesium dichloride was added slowly to ethanol in a 500-mL round-bottom flask fitted with condenser inert gas purge and placed on oil bath above hot plate-cum-magnetic stirrer. Mixture was stirred for 10 min by magnetic spin bar at 1000 rpm at room temperature to ensure complete dissolution. Vanadium triisopropoxide was added slowly by a syringe to the magnesium dichloride solution at room temperature. Solution color changed from transparent to yellow on addition of vanadium triisopropoxide. Calculated amount of caustic solution in deionized water was slowly mixed with solution by dropping funnel for hydrolysis. Reaction mass was heated to 70 ° C and hold for 2 h to ensure complete hydrolysis. Formation of white/milky solid was observed on completion of reaction. Solution became thick due to formation of magnesium hydroxide with increase in time. After 2 h of reaction, solution was cooled to room temperature and kept overnight for 16 h to complete gelation/co-precipitation process. MGV nanoparticles were washed three times with deionized water and separated from solution using centrifuge at 4000 rpm for 5 min each time. In the case of MGVBM and MGVOM, acetonitrile was used to remove/extract ionic liquid for washing of solid followed by centrifuge separation. Solids were kept at 110 ° C for 2 h to evaporate free water as well as acetonitrile. Later, vanadium-doped magnesium hydroxide nanoparticles were heated at 400 ° C for 4 h in muffle furnace to remove excess solvents (deionized water, acetonitrile) and ionic liquid. Calcination converts vanadium-doped magnesium hydroxide nanoparticles into vanadium-doped magnesium oxide nanoparticles by the removal of water molecules. The reactant quantity was taken as shown in Table 2.
The morphology characteristics of the nanoparticles were studied by HR-SEM at 12.5 kV supply voltage and TEM at accelerating voltage of 200 kV. Energy dispersion spectroscopic analysis was examined by an FEI Inspect scanning electron microscope equipped with an energy-dispersive X-ray spectrometer analyzer (EDX, Oxford Instruments, INCAx-sight). The sample was quickly fixed on the carbon tape under nitrogen atmosphere and loaded in the chamber. The voltage and working distance were varied during the measurements. XRD measurement was taken using Bruker D8 Advanced X-ray diffractometer. Step size of XRD was 0.02° from 5° to 70° , and time per step was 12 s. Analysis of XRD was performed using TOPASv 3.0 software supplied by Bruker AXS. Magnesium hydroxide and vanadium-doped magnesium hydroxide nanoparticles dried at 110 ° C for 4 h were evaluated by thermal gravimetry analysis (TGA) at a heating rate of 20 ° C/min from 60 to 700 ° C, and weight loss with respect to temperature was measured.
Photocatalytic experiments were performed in tailor-made cylindrical cell (i.d. 5.5 cm) of borosilicate glass. The 125-W integrally filtered low-pressure mercury UV tubes emitting near UV radiation (300-400 nm) with a peak at 365 nm were located in middle of the reactor. Cooling water was circulated in jacket to avoid heating of borosilicate glass avoiding excessive heating and cracking. Methylene blue was taken as model compound for degradation study. A 400 mL methylene blue solution (10 ppm concentration in demineralized water) was taken in reactor followed by addition of synthesized nanoparticles at 50 mg (125 mg/L). Before solution was put into reactor, mixture was sonicated for 1 h to ensure proper mixing and dispersion of micro-/nanoparticles. UV irradiation was started, and the samples were taken every 30 min up to 90 min. The samples were centrifuged for the separation of solids, and concentration of methylene blue was measured by UV visible spectroscope (Perkin make) by measuring solution absorbance. Methylene blue indicates that maximum absorbance peak at 665 nm and reaction progress can be judged based on reduction absorbance peak at 665 nm. Visible lamp (100 W, tungsten) was used as a radiation source for MB degradation. The distance between the radiation source and reactant was fixed at 10 cm. The MB concentration and the nanoparticles dosage were kept the same as UV light irradiation study.
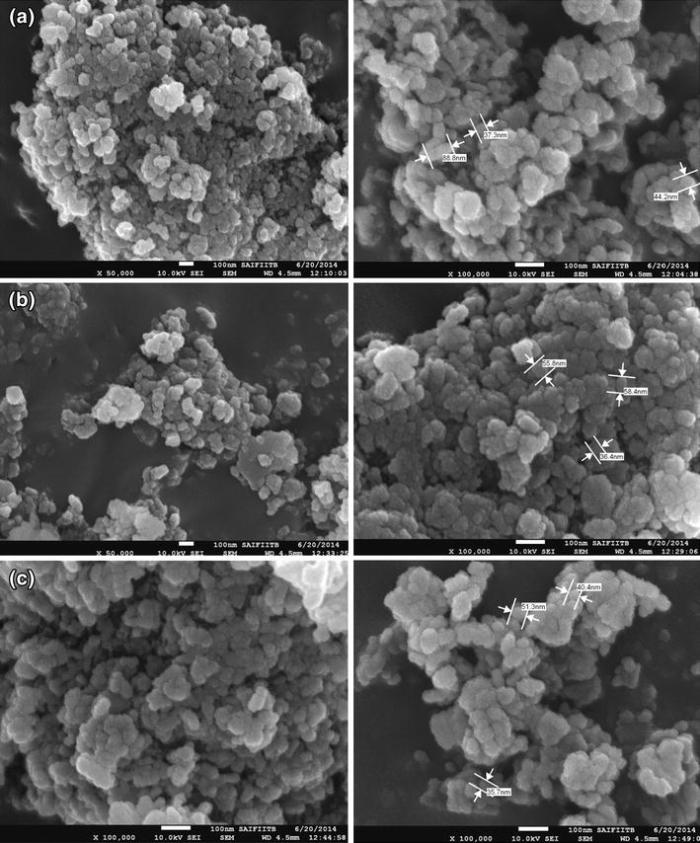
In order to understand the effect of ionic liquid on the characteristics of magnesium hydroxide nanoparticles, they were synthesized with and without ionic liquid and heated at 400 ° C for 4 h. HR-SEM (Fig. 2a) indicates MGC nanoparticles (without ionic liquid), while Fig. 2b, c exhibits MGBHC and MGOMC nanoparticles (with ionic liquid). The 40-nm to 80-nm MgO nanoparticles are formed in all three cases. Sizes of the nanoparticles are not influenced by the use of ionic liquids for synthesis of magnesium hydroxide particles. However, the nanoparticles are little more compact and uniform when the ionic liquids are used for synthesis.
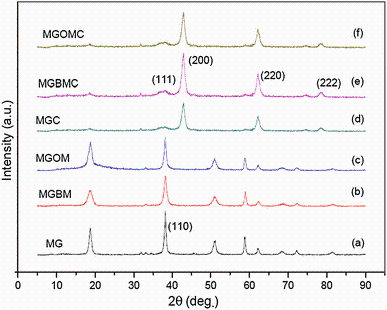
XRD experiment was performed for magnesium hydroxide nanoparticles as well as MgO nanoparticles to understand crystalline plane and to ascertain formation of MgO. Characteristic peaks at 18° , 37° , 52° and 60° of 2θ are representation of magnesium hydroxide. Peak at 37° represents (111) plane of magnesium hydroxide (Fig. 3a-c). The peaks are observed in XRD pattern of 2θ values of 38° , 43° , 62° 75° and 80° (Fig. 3d-f), which represent (111), (200), (220), (311) and (222) plane of magnesium oxide obtained after calcination of magnesium hydroxide at 400 ° C for 4 h [20].
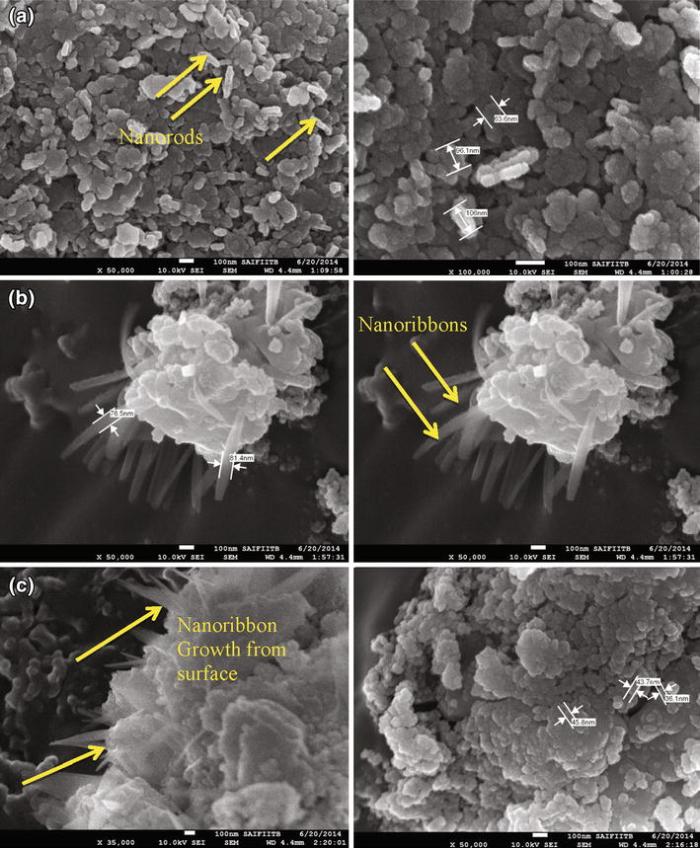
HR-SEM analysis of vanadium-doped magnesium oxide nanoparticles indicated two types of morphologies. Most of the nanoparticles are spherical in shape; however, few nanorods can also be seen on the surface. Nanospheres are in the range of 40-80 nm for all three synthesized vanadium-doped magnesium oxides— MGVC, MGVBMC and MGVOMC. Figure 4a shows nanorods, which are attributed to the presence of vanadium pentoxide, while nanospheres can be ascribed to the presence of magnesium oxide based on morphology study of magnesium hydroxide in the earlier section. Figure 4b, c also indicates two types of morphologies, namely nanospheres and nanorods. However, compared to MGVC nanoparticles, MGVBMC and MGVOMC nanorods are in the shape of nanoribbon and are comparatively longer. Nanosphere characteristics have remained the same for all three vanadium-doped magnesium oxides. Change in morphology from nanorod to nanoribbon (width < 80 nm) is attributed to the use of ionic liquid for synthesis of the nanoparticles. The [OMIM][BF4]-assisted MGVOMC indicated thin nanoribbons, while [BMIM][BF4]-assisted MGVBMC showed relatively thick nanoribbons. Nanoribbons are found to be originated from surface of clusters of the nanospheres.
Morphology and crystalline nature of magnesium oxide (MGC) and vanadium-doped magnesium oxide (MGVBMC) were further investigated by TEM (Fig. 5). MGC and MGVBMC were chosen to understand the change in morphology due to vanadium doping in the presence of ionic liquid. MGC nanoparticles revealed fine lattice with polycrystalline behavior (Fig. 5a). MGVBMC exhibited two fine lattice patterns (Fig. 5b) as also observed in HR-SEM. Nanoribbons were found to have 30-40 nm in size due to the vanadium doping and were integral part MgO lattice.
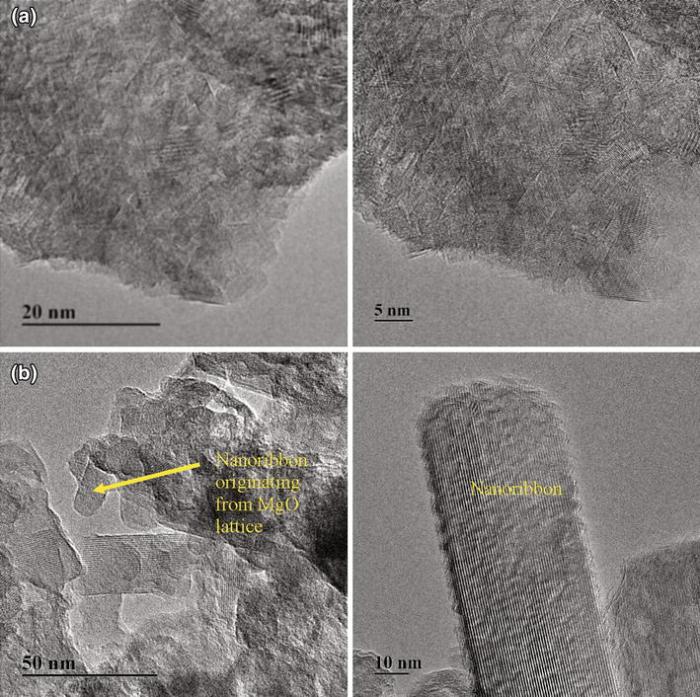 | Fig. 5 TEM images of magnesium oxide (MGC) and [BMIM][BF4]-assisted vanadium-doped magnesium oxide (MGVBMC) |
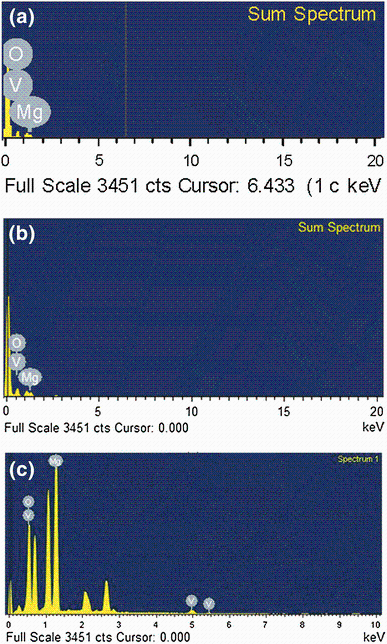
Morphology of vanadium-doped magnesium hydroxide MGVOM (before calcination) was further studied by HR-SEM. The particle size was found to be more than 200 nm, and most of the particles are found as agglomerated or fused mass (Fig. 6). These particles are compact and do not have any voids. This indicates that the particles were disengaged and more porous particle network was formed on heating 400 ° C for 4 h. Further, the particle size also reduced from > 200 nm to < 80 nm on calcination. Vanadium pentoxide presence on the surface of MGVC, MGVBMC and MGVOMC was determined by EDX analysis (Fig. 7). Vanadium along with magnesium and oxygen could be observed on the surface confirming formation of vanadium pentoxide-doped magnesium oxide. MGVC, MGVBMC and MGVOMC showed 3.9, 5.1 and 6.0 wt% vanadium, respectively. Use of ionic liquid has increased vanadium content in the synthesized nanoparticles. This may be due to better dispersion of the nanoparticles, allowing more surface of magnesium hydroxide exposed for co-precipitation of vanadium during gelation process.
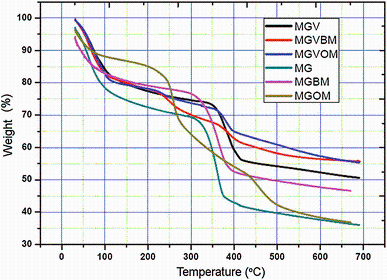
Magnesium hydroxide and vanadium-doped magnesium hydroxide were subjected to TGA analysis to understand weight change as a function of temperature rise (Fig. 8). The (11-21) wt% mass reduced from 50 to 250 ° C for magnesium hydroxide when [BMIM][BF4] and [OMIM][BF4] were used. About 21 wt% reductions were observed for all vanadium-doped magnesium hydroxide (MGV, MGVBM and MGVOM). Higher weight loss was observed for doped magnesium hydroxide compared to as-such magnesium hydroxide. Weight loss at 50-250 ° C can be attributed to loss of bound water from magnesium hydroxide and bound ethanol from vanadium-doped magnesium hydroxide. Higher amount of bound ethanol in case of doped particles is the reason for more weight loss.
On increasing temperature from 250 to 450 ° C, about 28-36 wt% loss was observed in the [BMIM][BF4] and [OMIM][BF4] used magnesium hydroxide (MG, MGBM, MGOM), which was higher than (15-22) wt% loss for that of vanadium-doped magnesium hydroxide (MGV, MGVBM, MGVOM). Magnesium hydroxide undergoes decomposition from 300 to 320 ° C, and decomposition completes at 450 ° C. Less weight loss in the vanadium-doped magnesium hydroxide is due to the presence of vanadium pentoxide, which does not undergo decomposition from 250 to 450 ° C like magnesium hydroxide. Considerable weight loss is not found when ionic liquid was used for synthesis compared to without ionic liquid. This is due to the effective removal of ionic liquid by acetonitrile washing during synthesis. Very less weight loss was observed from 450 to 650 ° C.
UV spectra of methylene blue indicate maximum at 665 nm. Peak absorbance was measured at 30, 60 and 90 min by UV-Vis spectrophotometer. In the case of control study (without nanoparticles), absorbance peak intensity reduced from 1.670 to 0.868 in 60 min, indicating 48% reduction. Absorbance peak intensity further reduced from 1.670 to 0.768, indicating 54% reduction in 90 min. It means that 48 and 54% methylene blue could be degraded in 60 and 90 min without the use of the nanoparticles (Fig. 9a). The absorbance value of methylene blue at 665 nm is described in Table 3. About 68% reduction in methylene blue concentration was observed in 30 min when MGC (Fig. 9b) was used as photocatalyst. Methylene blue concentration decreased by 93% (MGOMC) and 95% (MGBMC) for magnesium oxide nanoparticles synthesized in the presence of [OMIM][BF4] and [BMIM][BF4] (Fig. 9c and d). Use of ionic liquid for MgO synthesis has enhanced photocatalysis efficiency probably due to formation of more compact nanoparticles. Performance sequence was MGBMC > MGOMC > MGC > blank.
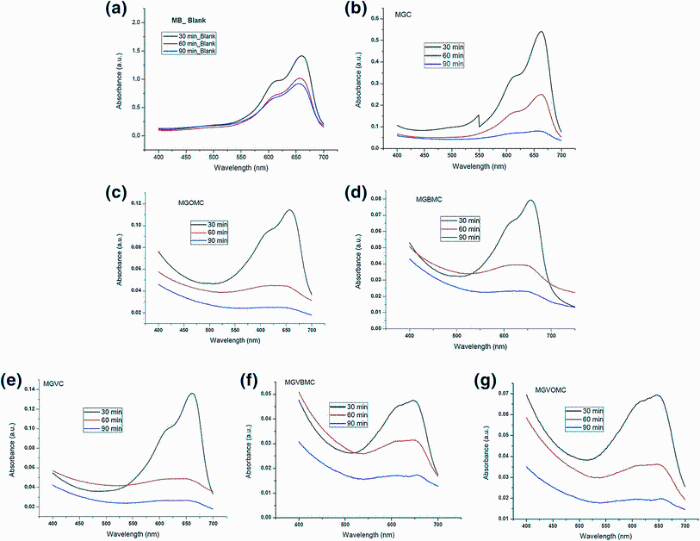 | Fig. 9 UV-Vis absorbance spectra for methylene blue photodegradation: using magnesium oxide a blank, b MGC, c MGOMC, d MGBMC and using vanadium-doped magnesium oxide e MGVC, f MGVBMC, g MGVOMC |
| Table 3 Absorbance value of methylene blue (MB) during photocatalysis study |
Further, when vanadium-doped MgO was evaluated, it was noticed that methylene blue concentration decreased by 92% for MGVC (vanadium-doped MgO was synthesized without ionic liquid, Fig. 9e). Methylene blue concentration further reduced by 98% (MGVBMC) and 95% (MGVOMC) when ionic-assisted vanadium-doped MgO was used (Fig. 9f, g). It can be seen that there was 35-45% increase in nanoparticle efficiency due to doping of vanadium pentoxide, which was further increased due to use of ionic liquid. Performance sequence of doped nanoparticles was MGVBMC > MGVOMC > MGVC > blank. Overall performance sequence observed as photocatalyst is MGVBMC > MGVOMC > MGBMC > MGOMC > MGVC > MGC > blank.
Maheshwari and Venkatachalam [21] reported the morphological combination of the nanorods and the nanosphere of titanium dioxide synthesized by sol-gel method and alkali hydrothermal method, which lead to the improvement in dye-sensitized solar cell. In our present study, we observe the improvement in photocatalytic degradation of methylene blue due to the combination of two kinds of morphologies was contributed by magnesium oxide and vanadium pentoxide. Several scientists have showed that doping of MgO can enhance the photocatalytic activity. Anilkumar et al. [22] disclosed that Fe+3-doped MgO increased photocatalytic decolorization of methylene blue under UV light irradiation. MgO and 4% Fe-doped MgO showed about 10 and 35% reduction, respectively, in 30 min using 30-W UV light source at 10 ppm MB solution at 60 mg nanoparticles dosing. Shahid et al. [23] modified magnesium oxide by sodium and titanium for achieving higher photodegradation activity of methylene blue by UV light irradiation. CaO/MgO nanoparticles showed 44% reduction in MB concentration in 80 min at 5 mg dosing. Yan et al. [24] showed about 99% MB reduction with square/labyrinth shape synthesized titanium dioxide as well as 98% MB reduction with P-25 commercial titanium dioxide nanoparticles at 400 W UV light and 10 ppm MB concentration. Our present study showed 98% reduction with 5.1% vanadium-doped MgO using 125-W UV light at 50 mg nanoparticles loading and 10 ppm MB concentration. This shows that our results are in good agreement with the reported literature on MB degradation using doped MgO or TiO2.
MGVBMC and MGVC nanoparticles were further evaluated by reusability study as these nanoparticles have shown good photocatalytic activity under UV light irradiation. Methylene blue concentration was adjusted to original level at the end of each cycle. UV absorbance data showed 98% reduction in MB concentration in 30 min at the end of first cycle. The second, third and fourth cycles of MGVBMC showed 97, 96 and 94% reduction in MB concentration, respectively (Table 4). MGVC nanoparticles showed 92%, 87%, 82% and 75% reduction in MB concentration in 30 min at the end of first, second, third and fourth cycles, respectively (Table 4). This shows that [BMIM][BF4]-assisted vanadium-doped magnesium oxide nanoparticles (MGVBMC) have good potential for reusability probably due to the better crystal structure and vanadium packing in MgO structure. However, vanadium-doped magnesium oxide (MGVC) efficiency reduces after the fourth cycle. Reusability study of P-25 titanium dioxide supported on zeolite indicated good stability of photocatalyst and reusability for eight times for a pesticide degradation [25]. MGVBMC has also shown good stability for reuse even without supporting it on any carrier.
| Table 4 Reusability of vanadium-doped magnesium oxide for methylene blue (MB) degradation under UV light irradiation |
MB degradation under visible light irradiation was conducted under control experiment (without use of the nanoparticles). UV-Vis absorbance of MB indicated 25% reduction in 6 h for control experiment as shown by reduction of absorbance from 1.67 to 1.26 (Fig. 10a). MGVBMC, MGVC and MGC degrade MB by 87%, 79% and 73% in 6 h of visible light irradiation. UV-Vis absorbance spectra of all the three nanoparticles are shown in Fig. 10. MB degradation data thus indicated that vanadium doping of MgO and further ionic liquid-assisted vanadium-doped MgO improved nanoparticles efficiency under visible light. Literature [26] suggested that MgO nanoparticles have band gap of 7.8 eV. Doping of MgO with transition metal such as Ti or Sn decreases band gap energy to 3.5-4.5 eV [26]. Reduction in band gap increases photocatalytic activity due to increased electron emission by the defects in MgO [27].
 | Fig. 10 Visible light absorbance spectra for methylene blue photodegradation: using magnesium oxide a blank, bMGC and using vanadium-doped magnesium oxide c MGVC, d MGVBMC |
Magnesium oxide and vanadium-doped magnesium oxide nanoparticles were successfully synthesized using magnesium dichloride and vanadium oxytriisopropoxide as precursor with and without ionic liquid by sol-gel method. Nanoparticles (40-80 nm size) having spherical and rod/ribbon shape morphology evolved owing to magnesium oxide and vanadium pentoxide as confirmed by HR-SEM and TEM. Presence of vanadium on magnesium oxide was also ascertained by EDX analysis. Photocatalytic activity for MB degradation under UV light irradiation increased due to ionic liquid-assisted doping of vanadium pentoxide on MgO, which further showed better reusability. MB degradation under visible light irradiation also showed improvement after ionic liquid-assisted doping of vanadium pentoxide on MgO compared to MgO.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|