In the recent years, biological nanostructures coatings have been incorporated into orthopedic and dental implants in order to accelerate osseointegration and reducing surgical restrictions. In the present work, chemical etching, anodization and metal doping surface modification methods were integrated in one strategy to fabricate innovative titanium surfaces denominated by titanium nanoporous, anodized titanium nanoporous, silver-anodized titanium nanoporous and gold-anodized titanium nanoporous. The stability properties of nanostructures-coated surfaces were elucidated using electrochemical impedance spectroscopy (EIS) after 7 days of immersion in simulated biological fluids. Morphology and chemical compositions of new surfaces were characterized by scanning electron microscope and energy-dispersive X-ray analysis. The EIS results and data fitting to the electrical equivalent circuit model demonstrated the influence of adsorption of bovine serum albumin on new surfaces as a function of protein concentration. Adsorption process was described by the very well-known model of the Langmuir adsorption isotherm. The thermodynamic parameter Δ GADS (-50 to 59 kJ mol-1) is calculated, which supports the instantaneous adsorption of protein from biological fluids to new surfaces and refers to their good biocompatibility. Ultimately, this study explores new surface strategy to gain new implants as a means of improving clinical outcomes of patients undergoing orthopedic surgery.
Titanium and titanium alloys have been broadly employed as implantation materials due to their favorable properties such as lower modulus, good tensile, excellent corrosion resistance and biocompatibility, so they are well suited to be used for orthopedic and dental applications as implants. At an early stage of implantation, titanium and its alloys considered bio-inert and they cannot bond with bone directly because they isolated from the surrounding bone by fibrous tissue, which leads to implant dislocation and premature loosening. Over the last 10 years, the research was directed to improve the titanium surfaces in the nanoscale regime to simulate the natural bone and to be able to demonstrate excellent osseointegration at the bone-implant interface [1, 2, 3].
In the recent years, there has been increasing interest in the formation of nanostructures bioactive coating based on titanium by different surface treatment methods, such as acid and alkali treatment [4, 5, 6] and electrochemical oxidation [7], which will improve osseointegration of titanium implants. The recent reports indicated that high surface area of the nanostructure implant surface topography helps to increase the corrosion resistance, provides available sites for protein adsorption [8], inhibits the bacterial adhesion [9] and thus enhances the cell-implant interaction for the successful healing process.
Since a decade or so, the electrochemical anodization process is considered a low-cost surface treatment method to prepare a homogenous porous, adherent and rough titania (TiO2) coating, which will induce apatite formation in living environment or simulated body fluid to enhance bioactivity [7]. The formation of thick, adherent and micro/nanoporous TiO2 films on titanium by anodic oxidation depends on the nature and concentration of the anodizing electrolyte, voltage, current density and temperature [10]. Similarly, wet chemical etching, another low-cost surface treatment method, has attracted the attention of the researchers and their efforts to improve it due to its efficiency to fabricate nanostructure surfaces [11].
Recent studies have shown that metal incorporation into the nanostructure titanium surface is considered global objective [12] to form multifunctional coating surfaces with multi-characteristics such as excellent corrosion resistance, high thickness uniformity with monolayer accuracy [13], capable of the rapid osseointegration with the surrounding host tissues [14] and a great ability to resist bacterial invasion [15].
Applications of TiO2 nanostructure as carriers for secondary materials have successfully been used to deposit biomolecules such as Cu [16], Ag [17], Au [18] and Ca-P particles [19, 20] to form passive/active coatings promoting the bone healing of the implanted surfaces and preventing microbial infection [21].
Bone, like other tissue, made up of blood vessels where serum albumin (SA) is the most abundant protein in human blood and represents 52%-62% of the total protein, which plays an extremely important role to maintain the osmotic pressure and transporting a wide variety of compounds including fatty acids, metals, amino acids, steroids and drugs [22] into all parts of the body. When a biomaterial is implanted into the bone, its surface is immediately covered with blood and serum proteins that are considered first cellular responses to the implant surface and it is expected to lead to faster growth of cells and stabilization of the implant. Surface chemistry and topography are known as two of the most important factors affecting biological reactions and their modifications, and their effect on protein adsorption and cell adhesion has been extended [23, 24, 25, 26, 27, 28, 29]. The functional properties of titanium implant, such as good fast osseointegration, and antibacterial properties can be improved by incorporating some particles such as Ag and Au into TiO2nanostructures coatings via simple surface fabrication process.
This paper reports new surface modification strategy based on three consecutive surface treatment methods aimed to activate the corrosion resistance of titanium implant in biological fluids. To evaluate the adsorption effect of the bovine serum albumin (BSA), which is similar to SA, on all modified surfaces compared with Ti, electrochemical impedance spectroscopy was used. The adsorption parameters are calculated to predict the biocompatibility response. SEM/EDX surface analytical techniques were used to characterize the morphology and chemical composition of new surfaces before and after the process of protein adsorption.
Titanium foil (Sigma-Aldrich Chemie GmbH, Riedstr.2D-89555 Steinem 49 7329 970) with 0.25 mm thick and 99.7% purity was used in this study as base substrates. Prior to each experiment, the samples were mechanically polished using progressively finer grades of silicon carbide papers (400-, 600-, 800-, 1000- and 1200-grit). After polishing, the samples were successively cleaned with acetone, alcohol and deionized water in an ultrasonic bath. After cleaning, modification steps were applied to prepare the new nanostructure surfaces in the following sequence: (1) The Ti sample was etched in concentrated lactic acid and ammonium fluoride bath for 4 h at room temperature to prepare titanium nanoporous sample, called TNP. (2) TNP sample was anodized in a safe chloride/glycerol bath at 20 V for 2 h at room temperature to prepare anodized titanium nanoporous sample, called ATNP. Anodizing process was performed with a regulated DC power supply (Sci-tech RDC 300 2T) in two-electrode cell, where a square sample of TNP (with area 1 cm2) was used as an anode and a platinum plate of area 3 cm × 3 cm was used as a cathode. (3) ATNP was immersed for 30 min in 0.1 mol/L AgNO3 solution at room temperature to prepare ATNP supported with silver nanoparticles (AgNPs), called Ag-ATNP. (4) ATNP was socked for 30 min in HAuCl3 solution at room temperature to prepare ATNP supported with gold nanoparticles (AuNPs), called Au-ATNP.
All solutions were freshly prepared from analytical grade chemical reagents and were used without further purification. The solvent was mostly deionized water or, in some cases, glycerol. The electrochemical behavior of all modified samples was studied in Hank’ s balance salt solution (HBSS) (NaCl (8.0 g/L), NaHCO3 (0.359 g/L), NaH2PO4· 2H2O (0.060 g/L), KCl (0.400 g/L), KH2PO4 (0.60 g/L) and d-glucose (1.0 g/L)) to study the biological activity. BSA is a well-characterized protein with a molecular weight of 66, 430 g/mol [26]. BSA concentrations varied from 0 to 6 g/L were used. The pH value of HBSS electrolyte solution was 7.4 at 37 ° C, and the tests were conducted in aerated environments.
The surface morphologies of new surfaces were characterized by using scanning electron microscopy (SEM, JEOL JSM 6390 LA). The compositions of such modified substrates were determined using ZAF software to quantify the energy-dispersive X-ray spectroscopy (EDX) spectra obtained by an EDX attachment (JEOL EDS EX-54175JMU) on the JEOL SEM [27, 28].
EIS measurements were used to estimate the bioactivity properties and investigate the corrosion resistance of the presently fabricated titanium surfaces TNP, ATNP, Ag-ATNP and Au-ATNP compared with uncoated Ti in HBSS free and HBSS containing a different BSA concentration. The EIS is a nondestructive sensitive technique which enables the detection of any changes occurring at the electrode/electrolyte interface. All EIS measurements were carried out using a potentiostat AUTOLAB (PGSTAT 30 with FRA modules, Ecochemie) in the same compartment three-electrode cell, which is discussed previously. All EIS spectra were obtained by applying the open circuit potential at a frequency range of 0.1 Hz-100 kHz to evaluate the structural stability of all modifies titanium surfaces in the determined electrolyte solution. The impedance spectra are displayed as Nyquist and Bode diagrams and were interpreted using a fitting procedure fit program ANOVA 1.8 program in the range of frequency. The criteria used in estimating the quality of the fitting were evaluated: first, with the lower Chi-square value and secondly, with the lower estimation errors for all the components [27, 28].
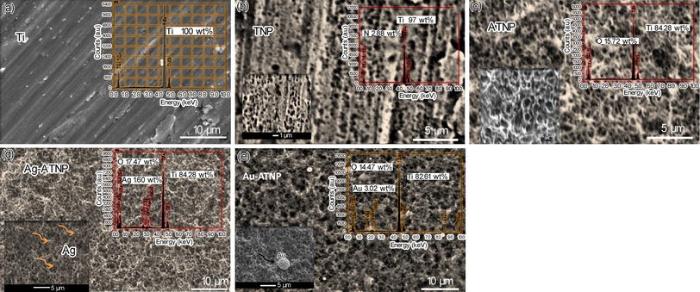
The following section studies the surface analysis of new nanostructure modified Ti surfaces to clarify how our surface strategy can change the morphology of Ti surface. Figure 1 shows the SE micrographs with EDX analysis of bare Ti and new coating titanium samples with TNP, ATNP, Ag-ATNP and Au-ATNP. As shown by Fig. 1a, the SEM image of Ti surface is similar to the mechanically polished surface, which represented the typical morphology of the native oxide film, with thin and non-porous structure [28]. The EDX analysis of Ti surface confirmed that the major element present is Ti. Figure 1b shows the SE micrograph of TNP prepared after etched pure Ti sample with lactic acid/ammonium fluoride bath. TNP surface was observed as a non-homogeneous porous surface with scattered pores. The EDX analysis of TNP surface represented that the etching solution may be considered as a source of nitrogen which can incorporate easily to the surface, and this result may add further features to TNP surface. SE micrograph of ATNP (Fig. 1c) shows that the anodization of TNP at 20 V for 2 h in the safe chloride bath at room temperature is considered as the optimum anodizing condition to form a spongy homogeneous nanoporous layer from TiO2. This observation is agreement with other reports [30]. The presence of 15 wt% of oxygen in the EDX analysis of ATNP surface can be explained due to the formation of TiO2 film. On the other hand, the homogeneity of the nanoporous structure of oxide film formed can be attributed to rapid access to the equilibrium point between the growth reactions of TiO2 film as described by
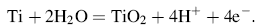
And the dissolution reaction [31] of it in the presence of chloride ions is described by
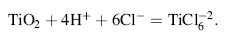
Figure 1d and e shows also the SE micrographs of Ag-ATNP and Au-ATNP samples, respectively. Spongy highly nanoporous titanium surface can be clearly observed, which greatly resembles the natural bone [32] with dispersed spherical particles from Ag and Au in nanoscale size. The presence of Ag and Au nanoparticles is confirmed by EDX investigation. The results indicated without any doubt that the anodization of TNP in HCl/glycerol bath produces a bioactive nanoporous TiO2 (17 wt% O2) surface which can attract Au and Ag NPs electrostatically from their solutions [33]. The low significant amount of Ag or/AuNPs (1-2 wt%) is considered as the most important observation. In the present work, the titania surfaces impregnated with Ag and Au noble metals will be expected to improve the biological activity of implanted surfaces through the inhibition of bacterial infections and reduce the cytotoxicity of the host tissues [21]. All results of EDX analysis are presented in Tables 1 and 2. The stability of these surfaces in simulated physiological solutions is investigated by electrochemical measurements in the following sections.
| Table 1 Chemical composition of Ti, TNP, ATNP, Ag-ATNP and Au-ATNP samples before immersion, based on ZAF software (in wt%) |
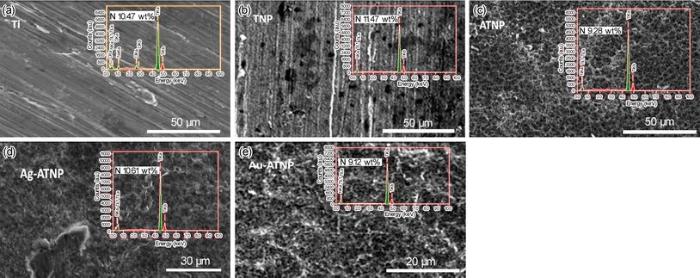
| Table 2 Chemical composition of Ti, TNP, ATNP, Ag-ATNP and Au-ATNP samples after immersion in HBSS solution containing 2.9 g/L BSA for 7 days at 37 ° C, based on ZAF software (in wt%) |
3.2.1 In HBSS Free
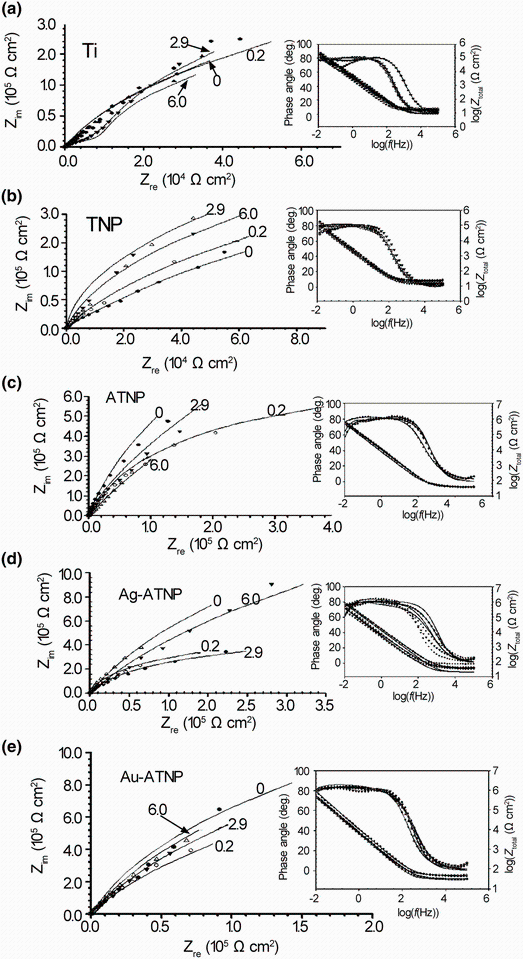
In this series of experiments, the in vitro bioactivity of new modified surfaces was evaluated by electrochemical EIS measurements to detect the interaction between their surfaces and HBSS. Impedance data are presented as Nyquist plot and Bode plots in Fig. 2 to compare between the electrochemical behaviors of uncoated Ti and nanostructural, coated TNP, ATNP, Ag-ATNP and Au-ATNP surfaces. All samples were examined after 7 days of immersion in HBSS at 37 ° C under open circuit potential. It is clear that the Nyquist plot can be characterized by the incomplete semicircle similar to a behavior of the near capacitive response. The semicircles diameters of all modified samples are higher than that of Ti, which pointed to the change in the morphology of the new surfaces by modification process. For Bode phase plots, the phase angle values up to the maximum value (~80° ) for all samples in the moderate frequency range. In this frequency range, all samples show capacitive behavior and the absolute impedance increased to the maximum value by decreasing the frequency. From this figure, the best significant result is observed where the highest diameters are recorded to Ag-ATNP and Au-ATNP surfaces. These behaviors reflect better corrosion resistance of impregnated titanium surfaces with noble metals than other surfaces (ATNP, TNP and Ti) [33]. The phase and impedance plot results show that the phase angle for all samples has two maxima, which means that the electrode/electrolyte interface is controlled by two time constants. The splitting in log(f) versus phase angle (θ ) diagram means that both the inner compact layer and the outer porous layer are contributing to the film growth kinetics [33]. The general feature of impedance plots specially for ATNP, Ag-ATNP and Au-ATNP is consistent with the passive film behavior which shows a phase angle approaching 90° over a wide range of frequencies (0.1 Hz-100 kHz).
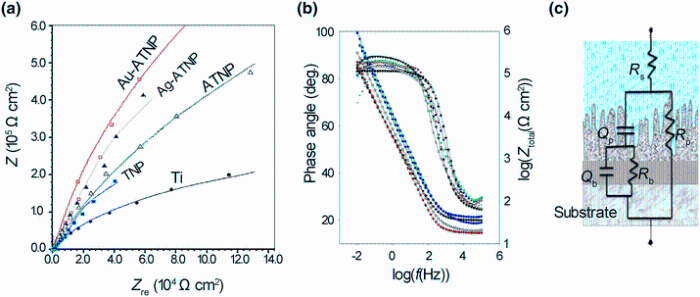 | Fig. 2 EIS spectra for TNP, ATNP, Ag-ATNP and Au-ATNP samples after 7 days of immersion in HBSS at 37 ° C, comparing with Ti: a Nyquist plots; b Bode plots; c equivalent circuit (Rs(RpQp)(RbQb)) |
The equivalent circuit shown in Fig. 2c was considered for fitting the experimental results in the frequency range of 0.1-100, 000 Hz. The values of the electrical circuit parameters were calculated by ANOVA 1.8 software. The correlation between the measured and calculated data is shown in Fig. 2a, b. It can be found that good match between the measured and simulated data was achieved. The magnitude of the electrical circuit components along with the Chi-square value (χ 2) is reported in Table 3. The typical Chi-square values are less than 1, indicating a satisfactory fit [34]. In this model, the Rp-Cp combination was introduced to account for the outer porous film, while the Rb-Cb combination was introduced to account for the barrier film. The highest Rp and Rb values are recorded as the order Au-ATNP > Ag-ATNP > ATNP > TNP > Ti. This result means that both inner and outer layers for ATNP are porous [35] and the increase in the Rp andRb can be attributed to the incorporation of ions from the HBSS to the outer and inner films. The lowering values of QP and Qb for all new surfaces confirm the success of our strategy to form thick nanostructure titania films. All values of the equivalent circuit parameters are presented in Table 3. From the impedance results, the charge transfer resistance (Rct) was calculated as the sum of (Rout) and (Rin). Thus, the semicircle diameter in the impedance spectrum equals the charge transfer resistance. A constant phase angle element (CPE = Q) is introduced to replace the capacitor and to account for the non-ideal behavior of the capacitive elements due to the heterogeneity which results from surface roughness produced from anodization followed by the metal deposition process [27]. Furthermore, the capacitance or Q could be related to film thickness according to the following expression [26]:
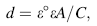
where d represents the thickness of passive layer, ε ° is the permittivity of vacuum (8.85 × 10-12 F/m), ε is the dielectric constant for the passive film, C is the capacitance obtained from fitting experimental results with the model proposed which is equivalent to the capacitance Q, and A is the effective surface area. The value of the dielectric constant for thin film depends on the experimental conditions, sample preparation and oxide film growth rate. This equation confirms the inverse relationship between the capacitance values and thickness of the film layer. In general, after 7 days of immersion, the charge transfer resistance of ATNP, Ag-ATNP and Au-ATNP is 6, 8 and 13 times higher than that of TNP or Ti, respectively. In specific, the charge transfer resistance of Ag-ATNP (5200 kΩ cm2) and Au-ATNP (8149 kΩ cm2) is higher than that of ATNP (4150 kΩ cm2). The results presented in Fig. 2 and Table 3 show how our strategy is responsible to change the topography of Ti surfaces and double their corrosion resistances more than one time.
| Table 3 EIS equivalent circuit (Rs(RPQp)(RbQb)) parameters for Ti, TNP, ATNP, Ag-ATNP and Au-ATNP implants surfaces after 7 days of immersion in HBSS containing 0-6 g/L BSA at 37 ° C |
3.2.2 In HBSS Containing BSA
Implants are usually surrounded by blood-rich tissues in the human body, and the serum proteins in the blood may also influence on their corrosion resistance. Thus, the stability of the new surfaces in simulated electrolyte with a BSA concentration range similar to the concentration as in human bodies (0-6 g/L) was studied. In these series of experiments, EIS measurements are used to follow up the corrosion resistance of all samples in the presence of BSA at different concentrations. Before the measurements, the samples were left stabilizing at open circuit potentials for 7 days in HBSS solutions containing (0-6 g/L) BSA. Impedance spectra are shown in Fig. 3 in the form of Nyquist diagrams and Bode plots for all coating titanium surfaces compared with Ti and are best fitting to the same equivalent circuit models, Fig. 2c. Fitting data are summarized also in Table 3, which emphasize the duplex nature of the coating passive films. The Nyquist plots of all samples, Fig. 3, are semicircles arcs which exhibit a typical passive state shape characterized by high impedance values with capacitive behavior [23]. On the other hand, two overlapped times constants can be considered in the impedance spectra, as shown in Fig. 3 also: the first one (Rp) corresponding to the outer heterogeneous coating passive film and the second one (Rb) corresponding to the inner barrier film. The charge transfer resistance (Rct) was also calculated as the sum of Rp and Rb and has been used for describing the influence of BSA concentration on the corrosion mechanism of ATNP, Ag-ATNP and Au-ATNP in HBSS at 37 ° C. In the presence of 2.9 g/L BSA, which is equivalent to the normal concentration of BSA in the human body, the charge transfer resistance of ATNP, Ag-ATNP and Au-ATNP is 4, 5 and 8 times higher than that of TNP or Ti, respectively. For another point of view, the charge transfer resistance of Ag-ATNP (4760 kΩ cm2) and Au-ATNP (7775 kΩ cm2) is higher than that of ATNP (3772 kΩ cm2). This result suggests that AgNPs and AuNPs play a significant role to enhance the adsorption of albumin from aqueous solution and increase the corrosion resistances [27], which confirm the success of the present strategy.
Because there are many diseases that may effect on human and cause a change in the proportion of BSA in the blood than normal ratio (2.9 g/L), in the present study the electrochemical behavior of new coating surfaces under different concentrations of BSA up and down 2.9 g/L was examined to take another evidence about their efficiency under critical biological conditions. Although Ag-ATNP exhibited a good electrochemical behavior in HBSS with BSA from 0 to 6 g/L and recorded high charge transfer resistances Rct (from 5200 to 4670 kΩ cm2 at 0 and 6 g/L BSA, respectively), ATNP surface recorded the higher charge transfer resistances than it especially at 6 g/L BSA. The expected antibacterial behavior of the Ag-ATNP due to the presence of AgNPs [36, 37] raises its efficiency more than ATNP. At lower and higher BSA concentrations, Au-ATNP is the best surface because it has the highest charge transfer resistance at low (0.2 g/L) and high (6.0 g/L) concentrations of BSA equal to 6820 and 8080 kΩ cm2, respectively, while the Rb values shifted from 149 to 280 kΩ cm2 by increasing the BSA concentration from 0 to 6 g/L; this finding means that the adsorption of BSA on Au-ATNP does not only enhance the resistance of the outer layer, but it also helps to increase the resistance of the inner layer. The incorporation of Au nanoparticles to ATNP surface raises the impact of modified titanium surface due to AuNPs, which is considered as anticancer agents [35, 38] and as an effective adsorbent for BSA molecules of the aqueous medium [39].
In this section of experiments, we follow up the change in the surface morphology of the fabricated new modified surfaces after 7 days of immersion in HBSS containing the normal concentration 2.9 g/L of BSA. Figure 4 illustrates the surface morphology and EDX analysis of novel coating surfaces in comparison with Ti. The presence of BSA in the solution activates the formation of a thin layer of nitrogen, which covers all surfaces by different mass percentages. This thin layer is due to the adsorption of BSA on the surfaces, which was confirmed by the EDX results. The presence of N on the surface may help to form the hard TiN layer, which increases the corrosion resistance of new coating surfaces [40]. On the other hand, this result might be explained that nanostructures titania surfaces can accelerate the adsorption of BSA [38]. The network sponge-like nanostructures of ATNP, Ag-ATNP and Au-ATNP play a key role to enhance the adsorption capacity of BSA adsorbed from the simulated solutions, and AgNPs and AuNPs extra-enhance the adsorption process [39, 40].
The equilibrium adsorption of BSA data on all present study samples can be described by the following Langmuir isotherm equation [41, 42, 43, 44, 45, 46, 47]
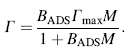
The Langmuir isothermal equation can be rewritten as:
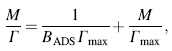
where M (mol/mL) is the equilibrium concentration of the adsorbate BSA in the bulk solution, Γ (mol/cm2) is the amount of protein adsorbed, i.e., surface concentration, Γ max (mol/cm2) is the maximum value of Γ (saturated surface concentration), and the parameter BADS reflects the affinity of the adsorbate BSA molecules toward the adsorption site.
The measured Rct values estimated from EIS measurements are equivalent to the amount of BSA absorbed on all samples. Since Rct is directly proportional to Γ [42], substitution of Rct by Γ gives the following rearrangement:
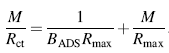
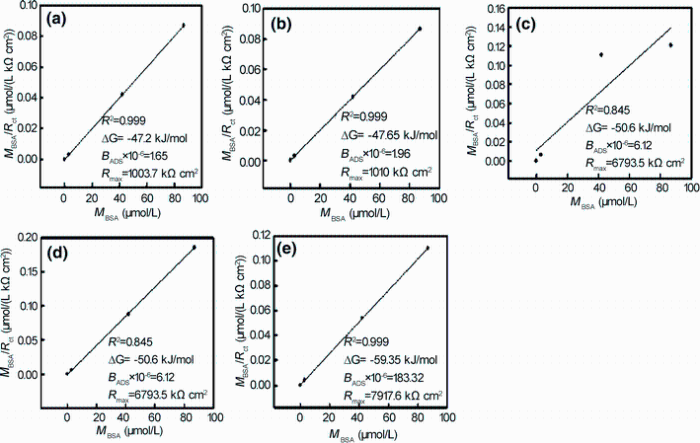
If the Langmuir isotherm is valid for an observed system, a plot of M/Rct versus concentration M should yield a straight line with parameters Rmax and BADS derived from the slope and intercept, respectively. As it can be seen from Fig. 5, adsorption of BSA is presented, and indeed, the M/Rct versus M dependence is linear. From the experimental and simulated values are shown that Langmuir isotherm could be successfully used to describe the adsorption of BSA on all modified surfaces and the best fit is obtained with an R2 value of 0.999. Therefore, Rmax and BADS values of the Langmuir equation were obtained from the linear regression in Fig. 5. Saturated surface concentration Γ max (substitution of Rmax) and affinity constant (BADS) for all coating surfaces are higher than those of uncoating Ti. These results reflect that the adsorption sites are less active on Ti than on ATNP, Ag-ATNP or Au-ATNP, leading to decreasing the adsorption efficiency. Also, the present results are in agreement with other reports where the electrostatic interaction between protein and surfaces is an important driving force for protein adsorption process [36, 41].
The Gibbs free energy function is the most common thermodynamic parameter used to predict how the study system changes spontaneously, especially at constant pressure rather than at constant volume. The parameter BADS, which indicates the affinity of the adsorbate molecules toward adsorption sites on the surfaces at a constant temperature related to the Gibbs free energy function, could be presented as [42]:
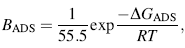
where GADS (kJ/mol) is the Gibbs free energy of BSA adsorption process on the modified Ti surface and the molar concentration of a solvent that in this case is the water (CH2O = 55.5 mol/mL). The Gibbs free energy values of adsorption of BSA on modified surfaces are ordered as: Au/ATNP > Ag/ATNP > ATNP > TNP > Ti. Such a high GADS (kJ/mol) negative value indicates strong spontaneous adsorption of BSA on all novel coatings implant surfaces [46]. Values of GADS for each new surface refer to the rate of BSA adsorption is depended on the surface topography of the implant and its chemical composition. These important results with the electrochemical and surface characterization results in this study support the present strategy to modify the surface by forming novel coatings convenient for orthopedic applications.
In the present work, a simple strategy for successfully constructing nanoporous titania via the combination of two surface treatments with metal deposition using silver nitrate and gold chlorides was illustrated. The produced nanoporous coatings can be considered the future generation of implant surfaces for enhancement albumin adsorption and cell response. The structures and resistances of new surfaces were investigated. Results show that TNP, ATNP, Ag-ATNP and Au-ATNP have the promising efficiency in orthopedic applications.
Grateful acknowledgment is made to Taif University represented in the Vice Presidency for Scientific Research on continuous support to provide all the capabilities to do this work.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|