Lots of the available literatures on hafnium in aluminum alloys are reviewed. The new binary Al-Hf phase diagram is simply assessed. Two ternary phase diagrams including Al-Hf-Zr and Al-Hf-Sc are accounted for, with emphasis on the aluminum rich part of the diagrams. The relationship between different structure Al3Hf and several different formation mechanisms including the probable phase transformation mechanisms is described. The continuous Al3Hf phase particles can serve as a grain refiner in the Al melt and a precipitate for controlling the grain structure of the alloy and a strengthening precipitate. A series of effects of Fe, Si, Zr, Sc and Li addition on the behavior of Al3Hf and Al alloys are given. Moreover, the effects of Hf on the microstructure and properties of Al alloys, such as hardness and creep, are reviewed. Finally, some views of Hf-containing Al alloys are summarized.
Aluminum alloys with good properties at elevated temperatures have a potential use in automotive and aerospace industries where a high strength/weight ratio is required. Many components in automobiles and aircrafts are subjected to high temperatures, e.g., engine parts, fuselage and heat exchangers [1, 2, 3, 4].
Historically, many efforts to develop high-strength, thermally stable Al alloys have sought alloying elements that exhibit both limited solid solubility and low diffusivity in Al. Based on diffusion-controlled coarsening theory, Adam [5] originally argued that dispersed phases formed from such alloying additions would be resistant to Ostwald ripening. Knipling et al. [6] concluded the criteria for developing castable, creep-resistant aluminum-based alloys with high temperature stability and strength, that alloying additions are (1) capable of forming a trialuminide strengthening phase, (2) low solid solubility, (3) low diffusivity in Al and (4) the ability for the alloy to be conventionally solidified. Several Al-based systems containing transition metal elements, such as Sc, Ti, Zr and/or Hf, for potential elevated temperature use were suggested because of low diffusivity and solubility of these elements in Al [7, 8]. These alloys derive their high-temperature properties from a wide range of intermetallic phases that formed during different solution treatments.
Plenty of reported studies have focused on scandium [9], zirconium, titanium, while hafnium in aluminum has been rarely investigated and reviewed. In this review, we will mainly focus on aspects of hafnium in aluminum alloys about the binary and ternary phase diagrams, the characteristics and transformation of Al3Hf phase, the influences of alloying elements on the precipitation of Al3Hf and the effects of Hf on the microstructure and properties of aluminum alloys.
Murray et al. [10] have firstly constructed an assessed phase diagram mainly based on the results published by Rath et al. [11], Pö tzschke and Schubert [12] and Tsyganova et al. [13], which is shown with dashed lines in Fig. 1. Due to large uncertainty in the experimental data, most phase boundaries were speculative. Some liquidus boundaries were strongly asymmetric, which is unlikely. The assessed phase diagram (Fig. 1) includes: (1) the liquid phase, L; (2) three terminal solid solutions, body-centered cubic [bcc(Hf)], close-packed hexagonal [cph(Hf)], and face-centered cubic [fcc(Al)]; and (3) seven intermediate compounds, AlHf2, Al2Hf3, Al3Hf4, AlHf, Al3Hf2, Al2Hf and Al3Hf. The lattice parameter data are listed in Table 1.
 | Fig. 1 Al-Hf phase diagram [16] |
 | Fig. 2 Al-rich portion of the Al-Hf phase diagram based on Rokhlin’ s results (points, solid lines) and the data in [11, 30] (dashed and dash- and dot-lines, respectively) |
| Table 1 Hf-Al lattice parameter data [10] |
Kaufman and Nesor [14] have performed the calculation of the Al-Hf system in 1975 and proposed a calculated phase diagram that was quite different from the experimentally determined one [11]. However, the model-calculated phase diagram and the thermodynamic properties conducted by Wang et al. [15] are in good agreement with most of the experimental data that are shown with solid lines in Fig. 1. Because the experimental data used in the thermodynamic modeling are essentially the same as those used by Murray et al., the accuracy of the phase diagram must be confirmed. Okamato [16] redrew the Al-Hf phase diagram by preprinting of Murray and Wang’ s results. Special points of the system including the calculated invariant equilibrium data are listed in Table 2. The results of the enthalpies of formation for the Al-Hf intermetallic compounds are compiled in Table 3. The concentration dependence of mixing enthalpies at 0 < XHf < 0.2 and 1790 ± 5 K for the Al-Hf binary system has been established for the first time, and appropriate quantities for the entire concentration range have also been modeled by Sudavtsova and Podoprigora [17] based on Meshel’ s work [18].
| Table 2 Comparison between selected and calculated invariant equilibrium data mainly from Wang et al. [15] and Murry et al. [10] |
| Table 3 Comparison of the enthalpies of formation for the Al-Hf intermetallic compounds, ∆ fH2980 (kJ g-1 atom-1) |
The Al-rich side of the Al-Hf phase diagram was studied by Rath et al. [11], Wang et al. [15], Zamotorin and Zamotorina [30] and Rokhlin et al. [31]. These studies indicated the peritectic character of the invariant reaction occurring in the Al-rich portion of the phase diagram at a temperature close to the melting temperature of pure aluminum, and the relative invariant reaction data are listed in Table 2. The solubility of Hf in both liquid and solid Al was determined and reported by three references [11, 30, 31], and the results are listed in Table 4. The solid solubility of Hf in aluminum at the peritectic temperature is approx. 0.5 wt%, which decreases as the temperature decreases below the peritectic reaction temperature, while the hafnium solubility in liquid aluminum increases gradually as the temperature increases above the peritectic reaction temperature. The Al-rich portion of the Al-Hf phase diagram is shown in Fig. 2.
| Table 4 Maximum solubility of Hf in Al at peritectic temperature |
There are quite less reports on the Al-Hf-X phase diagrams. Hafnium as an alloying element is often added together with Sc or Zr into aluminum alloys. In this case, one mainly focuses on the Sc and Zr effect on the material and on the effect of Hf on Sc- and Zr-formed precipitates. Based on the literature, we will mainly introduce two kinds of Hf-containing ternary systems, i.e., Al-Hf-Sc and Al-Hf-Zr.
The Al-rich part of the Al-Hf-Sc ternary phase diagram was systematically studied by Rokhlin et al. [34], subsequently compiled by Raghavan [35]. The Al-Hf-Sc isothermal section at 600 ° C near Al corner is given in Fig. 3. The partial isothermal sections at 500 and 400 ° C were essentially resemblance with that at 600 ° C, which are not shown here.
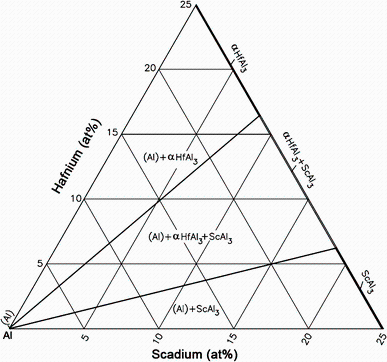 | Fig. 3 Al-Hf-Sc isothermal section at 600 ° C near the Al corner redrawn by Raghavan [35] based on the results from Rokhlin et al. [34] |
Using electrical resistivity measurements, optical microscopy and scanning electron microscopy with energy-dispersive X-ray spectrometry, the combined solubility of Sc and Hf and the phases in equilibrium in Al solid solution at 600, 500 and 400 ° C have been investigated. The results indicated that the Al solid-solution field in the Al-Hf-Sc phase diagram was contracted with decreasing temperature. Sc and Hf were shown to decrease the solubility of each other in a solid Al. The total solubility of Sc + Hf in a solid Al decreased gradually with increasing Sc/Hf ratio in the alloy.
Rokhlin et al. [36] also studied the Al-rich part of the Al-Hf-Zr ternary phase diagram. Using the similar method as for the Al-Hf-Sc, the obtained Al-Hf-Zr phase diagram showed an analogous character as the Al-Hf-Sc system, which could be due to the same group and similar chemistry of zirconium with scandium and hafnium. Taking into account the established extension of the Al single phase field and compositions of the ZrAl3 and HfAl3 compounds in equilibrium state, the partial isothermal sections of the Al-Hf-Zr phase diagram at 600, 500 and 400 ° C were constructed. Raghavan [37] redrew the Al-Hf-Zr isothermal section at 600 ° C near Al corner (Fig. 4), which represents the character of the sections at 500 and 400 ° C as well.
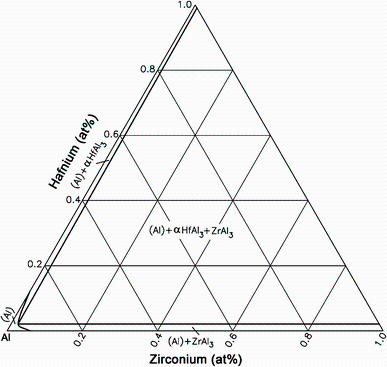 | Fig. 4 Al-Hf-Zr isothermal section at 600 ° C near the Al corner redrawn by Raghavan [37] based on the results from Rokhlin et al. [36] |
As the Hf and Zr have limited solubility in solid Al, they could hardly dissolved in ternary Al-Hf-Zr system. Zr and Hf significantly decrease the solubility of each other in solid Al with contraction of the Al single phase field with decreasing temperature. In addition, there is an unexpected and remarkable result that the solubility of Zr in Al3Hf and the solubility of Hf in Al3Zr are insignificant because both Al3Zr and Al3Hf have the same crystal structure, similar constitution of the outer electron shells of their atoms and close atomic radius.
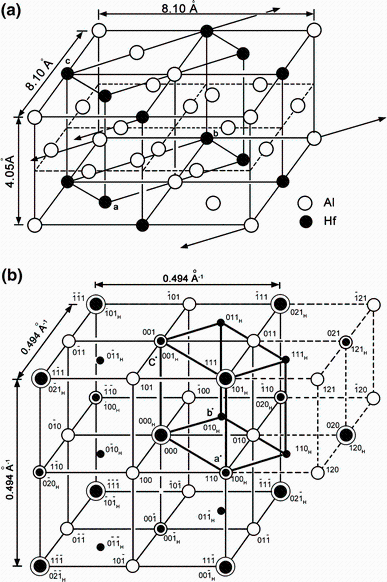
Based on the binary phase diagram of the Al-rich corner, the Al3Hf phase was firstly identified as the tetragonal D023 (8 atoms in the primitive cell) and D022 (16 atoms in the primitive cell) structure, an equilibrium phase, by Schubert et al. [21] and Boller et al. [25]. It was labeled as β -Al3Hf and α -Al3Hf, respectively (Table 1). The β -Al3Hf phase with Al3Zr type would transform into α -Al3Hf with Al3Ti type at a peritectic temperature about 650 ° C [12]. The lattice structure of the β -Al3Hf was also reported to be of the Cu3Au type as a metastable phase, nominated as L12 [28]. The structure can be described as ordered face-centered cubic (fcc), although in crystallographic terminology the Al3Hf structure is actually a simple cubic lattice with four atoms (one Hf and three Al atoms) associated with each lattice point.
The schematic diagrams of L12, D022 and D023 structures are shown in Fig. 5. The lattice parameters of Al3Hf from both the experimental measurements and calculations are collected and listed in Table 5. Of these structures, the latter two may be considered as superstructures of the simple cubic L12 cell. The D022unit cell consists of two L12 cubes stacked along the z direction, with a [1/2 1/2 0] antiphase shift between the cubes. In Zhdanov’ s [38] notation, D022 was 11¯ 1¯ where the bar indicated a domain of antiphase with respect to the previous one. Fisher and Selke [39, 40] introduced a more compact notation, which indicated the length of the domains between successive antiphase boundaries. The D023 structure consists of a stacking of four L12 cubes with the same antiphase shifting every two cubes, and D023 is 22¯ 2¯ . In Zhdanov’ s notation, L12 was < ∞ > . Colinet and Pasturel [41] have calculated the total energies of the compound Al3Hf in the L12, D022 and D023 superstructures using Vienna Ab-initio Simulation Package (VASP) based on the density functional theory (DFT) within the local-density approximation. The relative stability of the three structures in the last stage of the relaxation process was: E(DO23) < E(DO22) < E(L12).
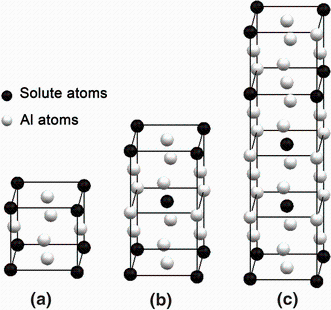 | Fig. 5 Unit cells of the three structures: a L12 structure, b D022 structure, ideal c/a = 2, c D023 structure, idealc/a = 4 |
| Table 5 Experimental and calculated lattice parameters of the Al3Hf phase in the L12, D022, D023 structures |
There are mainly three reported ways on the formation of the Al3Hf phase in dilute Al-Hf binary alloys.
1.Upon solidification of hyper-peritectic alloys (Hf > approx. 0.5 wt%), Al3Hf is the first phase to be formed, i.e., a primary Al3Hf phase.
2.Al3Hf can be precipitated discontinuously from supersaturated solid solution.
3.Al3Hf can be precipitated continuously (nucleation and growth) from supersaturated solid solution.
The latter two can be named as secondary Al3Hf phase.
According to the binary phase diagram, primary Al3Hf phase can be formed during solidification of all alloys with Hf content higher than approx. 0.5 wt%, i.e., higher than the peritectic composition.
The primary Al3Hf phase has a tetragonal lattice of D023 type. Brodova et al. [48] studied the Al-1.4 wt% Hf alloy cast at the conditions of various overheating of initial melt above liquidus and different cooling rates during solidification. The primary Al3Hf phase was observed in castings solidified with different cooling rates. Ryum [28] studied the Al-1.78 wt% Hf alloy casting from 1100 ° C in a copper chill mold placed on a water-cooled table. No primary intermetallics were observed within the grains, suggesting that hafnium was retained in solid solution during solidification. Hori et al. [29, 49, 50] have investigated the formation of primary Al3Hf in Al-X wt% Hf series alloys, which were casted in chilling. Under the cooling rate of 3 × 103 K/s, the primary Al3Hf was not appeared until the content of Hf was up to 3.5 wt%. It is curious that the primary Al3Hf phase was observed with angular structure in a splat cooled Al-6 wt% Hf alloy where the cooling rate was estimated to be 107 K/s, while the Al-6.4 wt% Hf alloy showed the presence of only a supersaturated solid solution with identical cooling rate [51]. Therefore, it can be concluded that the formation of primary Al3Hf phase in the Al-Hf alloys is dependent on both a cooling rate and an initial Hf content.
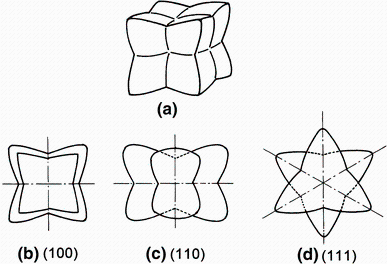
It was found by an electron microscope examination that angular structure did not consist of one phase but of a metastable Al3Hf having an L12 structure and α -Al. The metastable phase had a fine needle-like shape parallel to one of < 111> Al directions. In addition, the angular structure, which was surrounded by {100} plans and had eight processed orientations to < 111> Al directions, had a cube shape. The morphology and structure of such phase are shown and illustrated in Fig. 6 [29].
As described above, addition of hafnium often forms a supersaturated state in aluminum solid solution due to non-equilibrium solidification process. In particular, with a rapid cooling rate it can obtain quite high supersaturated Hf in solid solution. When the ingot is heat treated, supersaturated Hf will precipitate out from the solid solution and form new phase(s) together with Al and/or other alloying elements.
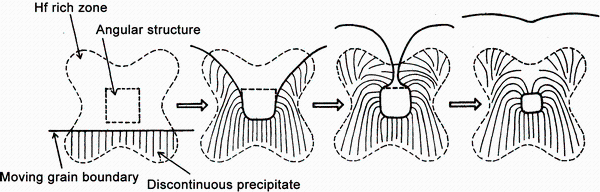
Discontinuous precipitation of the Al3Hf phase from supersaturated solid solution was often observed in the Al-Hf binary alloys [29, 43, 49]. The mechanism of discontinuous precipitation (sometimes referred to as cellular precipitation) was commonly described as a decomposition of supersaturated solid solution into α -Al and Al3Hf at a moving grain boundary [53].
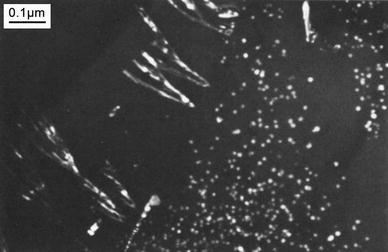
As the grain boundary moves, it leaved behind a characteristic fan-shaped or dendritic-shaped array of precipitates [52]. One can get the general idea of the characteristic shaped array of precipitates from the schematic representation of interaction of angular structure and grain boundary reaction (Fig. 7) drawn by Hori et al. [29].
Ryum [28] and Hori and Furushiro [50] observed only discontinuously precipitated precipitates in an Al-1.78 wt% Hf alloy annealed at 200 ° C and an Al-6 wt% Hf alloy at low temperature, respectively. This probably indicated that the diffusion of Hf in A1 was very slow, so that the precipitation took place at a measurable rate only in a discontinuous manner in which the Hf atoms diffuse in a grain boundary region [28]. After precipitation, the precipitates were very stable at 450 ° C over a long annealing time. Continuous and discontinuous precipitation of the L12-A13Hf phase in the Al-1.6 wt% Hf alloy aged at 300 ° C for 10 h is shown in Fig. 8.
 | Fig. 8 Continuous and discontinuous precipitation of the L12-A13Hf phase in Al-1.6 wt% Hf alloy aged at 300 ° C for 10 h (centered dark field TEM image taken along [100]) [43] |
In the Al-Hf case, the discontinuously precipitated Al3Hf particles with a metastable L12 type was found to be completely coherent with the α -Al matrix and bored a closed orientation relationship with the matrix [28, 51]. No strain field existed around the discontinuous Al3Hf in the Al-1.78 wt% Hf alloy which is different from that in the Al-Zr alloy [54].
Continuous precipitation of Al3Hf is a bulk decomposition process of supersaturated Hf in Al solid solution. In the isothermal case, it can be characterized by three stages, i.e., nucleation, growth and coarsening. It may be described as a diffusional reaction in a multi-component system in which atoms are transported to grow nuclei by diffusing relatively large distances in the parent phase. Thus, the mean composition of the parent phase changes continuously toward its equilibrium value. Continuous precipitation is usually a desired precipitation mode to obtain a fine dispersion of homogeneously distributed precipitates with a spherical type. These precipitates are coherent with the Al matrix and usually gathered in cluster as shown in Fig. 9 [49].
 | Fig. 9 Continuously formed spherical precipitates in chill cast Al-3.5 wt% Hf alloy aged at 450 ° C for 40 h |
Hori et al. [49] were not able to observe any continuous precipitation of the Al3Hf in an Al-1 wt% Hf alloy produced by splat cooling even after 2000 h aged at 450 ° C. This indicates that Al3Hf precipitates were extremely difficult to homogeneously precipitate in binary Al-Hf alloys. The temperature-time-precipitation diagram of solution-treated Al-1 wt% Hf alloy and chill cast Al-3.5 wt% Hf alloy is shown in Fig. 10.
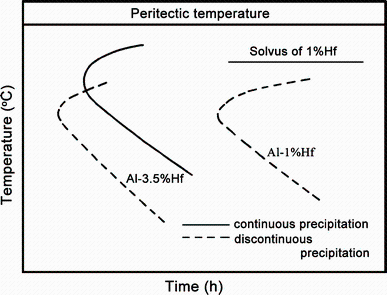 | Fig. 10 Temperature-time-precipitation diagram of solution-treated Al-1 wt% Hf alloy and chill cast Al-3.5 wt% Hf alloy (schematic) |
In addition, the grain boundary mobility of the splat quenched Al-Hf alloy was so high that discontinuous precipitation caused by a grain boundary reaction preceded continuous precipitation within grains [29].
The transformation of the Al3Hf phase including the structure and the morphology in the Al-Hf systems is dependent on annealing temperature and time. The decomposition of the supersaturated solid solution may thus be represented schematically in the following way [28]:
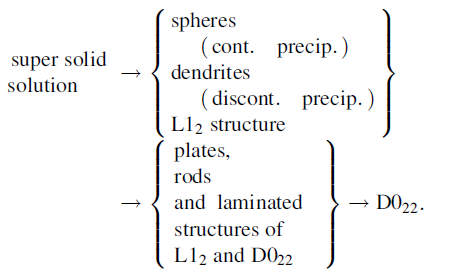
Decomposition of the supersaturated solid solution on annealing occurs by the formation of a metastable L12cubic Al3Hf phase, which, during heat treatment, will transform in either equilibrium D022 or D023 structures. The equilibrium structure will be obtained depending on the temperature and the way of formation of a metastable Al3Hf. The metastable L12-Al3Hf would transform into D022 type if it was formed from casting alloy, while it would transform into D023 type if it was formed by the mechanical alloying [42, 55, 56]. It must be noted that Srinivasan et al. [42] observed the transformation of L12 into D023 at 750 ° C and did not observe the transformation of D023 into D022 even by heating at higher temperatures which was further studied by Colinet and Pasturel [41] using the first-principle total-energy calculations. In addition, the transformation of the Al3Hf phases is quite different in the alloys with or without silicon.
Ryum [28] and Hori et al. [49] investigated the microstructure of the Al-1.78 wt% Hf alloy and Al-6 wt% Hf alloy with rapid solidification, respectively. They found that the metastable L12-Al3Hf structure was firstly formed from decomposition of supersaturated solid solution, which was obtained by a rapid solidification. Such precipitate had a spherical morphology or fan shape and distributed within the dendrites. These precipitates partially transformed into D022 structure with plates, rods and laminated shape when the metastable L12-Al3Hf was heated at 450 ° C for a long time. Some precipitates transforming into the laminated structure were developed along the < 110> directions of the matrix. It would be noted that in the Al-3.5 wt% Hf alloy all the precipitates hold the metastable Al3Hf and no further structural change took place during the subsequent aging, which may be due to the low cooling rate of 3 × 103 K/s. But pre-aging and pre-stressing were helpful for the transformation of L12 to D022 in the subsequent annealing of the alloy [49].
In addition, the imperfect D022 structure may be formed by a shear movement of a distance ½ < 110> on every other (001) plane of the L12 structure [28], and the perfect D022-Al3Hf structure needed the slight movements of the Hf and Al out of the (100) planes [57].
Hori et al. [58] have studied the effect of Si on the precipitates of Al-Hf alloys containing 0.1-0.5 wt% Si isothermally aged at 350-500 ° C. A new kind of intermediate (H) phase was proposed in the phase transformation from a metastable L12 structure to an equilibrium D022 structure. The observed sequence of the transformation is schematically shown in Fig. 11. In addition, Hori et al. [49] and Pandey and Suryanarayana [51] also observed that the metastable L12-A13Hf phase discontinuously precipitated during the early stages of decomposition transforms directly into the equilibrium tetragonal D022 structure in the silicon-free alloys.
Observed sequence of the transformation in the Al-3 wt% Hf-0.5 wt% Si alloy [49] A possible mechanism of phase transformation of Al3Hf from L12 to D022 during aging in a rapidly solidified Al-3 wt% Hf-0.3 wt% Si alloy was investigated by Furushiro and Hori [59]. The H phase was found to have a space group Pmmm (Fig. 12a). The transformation from one phase to the other could be explained by a periodic shear. Thus, a shear of 1/2[110](110)L12 on every second plane led to the H phase which transforms to D022 by a shear of 1/2[010](101) on every second plane (Fig. 12b).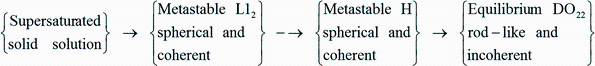
 | Fig. 12 a Apparent reciprocal lattice of the H phase, b the larger symbol represents the stronger reflection, thesmallest circles of the characteristic spots are placed at ½ < 110> in the reciprocal lattice of the L12structure |
In addition, it was found that the addition of Si in the alloys accelerated the continuous precipitation and decreased the time of age hardening, which was attributed to the acceleration of nucleation of spherical metastable particles by Si atoms within the grains [58]. On the other hand, Si in the alloys also suppressed the grain boundary reaction, i.e., depressing the discontinuous precipitation.
Hallem et al. also systematically investigated the dilute Al-Hf-Si alloys and found the similar results [53, 60]. Moreover, it was found that the addition of Si to the Al-Hf alloys leads a variation in grain size and precipitate morphology, which was dependent on the amount of Si addition. Relatively coarse and inhomogeneous AlHfSi particles with elongated shapes were formed in high Si-containing alloy at subsequent homogenization treatment. The formation of AlHfSi particles results in less Hf for the formation of small, coherent and spherical Al3Hf precipitates. As a consequence, the Si content would be kept below 0.15 wt% in order to avoid the formation of AlHfSi-particle according to Hallem’ s research on the wrought aluminum alloys [53].
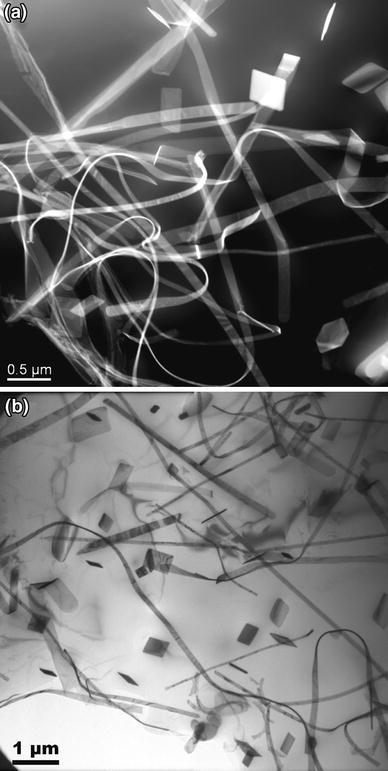
Most reported studies on hafnium in aluminum alloys have been focused on the wrought alloys. Jia and Arnberg [61] have investigated the microstructures of the Al-7 wt% Si-0.3 wt% Mg-0.5 wt% Hf-0.2 wt% Y cast alloy after solution treatment (Fig. 13). A novel phase was identified as Si2Hf-type phase by combining high-resolution transmission electron microscopy (HRTEM) and energy-dispersive spectroscopy (EDS) analyses. This “ nanobelt” precipitate was observed after elevated temperature treatment of 560 ° C, with extremely large length/width or length/thickness ratio and high density. From this work, one could see more about the effect of Si on Hf-related phases in aluminum.
Iron is one of additive elements or impurities in many commercial Al alloys, which may in some cases deteriorate or improve the properties of the alloys [62]. In the Zr-containing aluminum alloys, Fe acted as catalysts usually increases the rate of formation of Al3Zr particles [63], while in the Hf-containing aluminum alloys Fe improves the driving force and kinetics of Al3Hf precipitation [58].
The effect of iron on the Al-Hf alloys was systematically investigated by Hallem in his Ph.D. thesis work [60]. The amount of Fe in the range of 0.11-0.44 wt% did not show significant influence on the cast structure and the precipitation of Al-Hf alloys. Interestingly, the solubility of iron in aluminum was also increased from 0.02 wt% (according to the solidification model ALSTRUC) to 0.05 wt% with addition of Hf.
As mentioned in the section of ternary phase diagrams, considerable amount of Hf dissolved in the Al3Sc phase and thus a more correct denotation of the precipitation is Al3(Sc1-x Hf x ). In discussing the influence of the Sc content on the precipitation of Al3Hf, one is therefore actually discussing the difference between Al3Hf precipitation and Al3(Sc1-x Hf x ) precipitation.
Harada and Dunand [64] studied the microstructure of Al3Sc with Hf addition, leading to new ternary Al3(Sc1-x Hf x ) precipitates where x = 0.10, 0.25, 0.50. When the Hf concentration increases to 0.50, a new ternary Al3(Sc, Hf) phase is formed with D023 structure.
By using three-dimensional atom probe field ion microscopy (3D-APFIM), a Sc-rich cluster was found clearly in the Al3(Sc1-x Zr x ) phase in the Al-Sc-Zr alloys, but no Zr-cluster was observed [65, 66]. Zakharov and Rostova [67] found that a Al3(Sc1-x Hf x ) phase was formed when adding Hf to an Al-0.2 wt% Sc alloy. The dispersity of Al3(Sc1-x Hf x ) was higher than that of Al3Sc and less than that of Al3(Sc1-x Zr x ). It is also concluded that hafnium more than 0.15 wt% would be added to gain a better or satisfying effect. Compared with the previously investigations, Hallem [60] also found Sc-clusters, but no Hf-clusters observed in Al-Hf-Sc at 290 ° C in the reconstructed 3D-volume from atom probe experiments, which revealed that Sc controlled the initial stage of precipitation. Rokhlin et al. [34] indicated the progressive decrease of the total solubility of Sc and Hf in solid Al with increasing Sc/Hf ratio and decreasing temperature.
Zr is always found together with Hf in nature and is very difficult to be separated from Hf. Both Zr and Hf show many similarities in chemical and physical properties. Srinivasan and Chattopadhyay [68, 69] found a new metastable phase formed on annealing, which had a crystal structure related to the equilibrium orthorhombic Si2Zr. Gao et al. [70, 71, 72] found blocky AlZrSi intermetallic phase in the Al-Si-Zr alloy after annealing, respectively. Combined additions of Zr and Hf in Al alloys may form heterogeneously distributed Al3(Hf, Zr) coarse precipitates, but not Al3Hf precipitates, which improves the recrystallization temperature of aluminum alloys [73, 74]. Precipitation can actually be improved in Al-Hf-Zr alloys comparing with Al-Hf alloys [60]. The primary AlSi(Hf, Zr) phase formed during solidification was also found in Al-Si-Mg-Hf-Zr alloy in our unpublished work.
Rangel-Ortiz et al. [75] studied the microstructure evolution of the Al-1.2 wt% Li-0.8 wt% Hf alloys. The yield and ultimate tensile strength of the as-cast alloy were improved with the addition of hafnium and lithium. No A13(Li x Hf1-x ) precipitate in the as-cast materials was observed. Hf partly presented as an intermetallic precipitate improving the hardness of the as-cast Al-1.65 wt% Li-0.85 wt% Hf alloy [76]. According to Rangel-Ortiz et al. [77], the Hf-Li precipitates were more homogeneously distributed in Al-1.65 wt% Li-0.85 wt% Hf alloys and its size increased with increasing aging time.
Antipas et al. [78] studied the microstructure of spray formed Al-Hf and Al-Li-Hf alloys. Filamentary L12-Al3Hf for the Al-Hf alloy and spherical/filamentary L12-Al3(Li, Hf) for the Al-Li-Hf alloy were formed, respectively. The spherical particles were identified as the L12-Al3(Li, Hf) metastable phase, formed continuously [79, 80], which was consisted of a darker L12-Al3(Li, Hf) core surrounded by a lighter L12-Al3Li envelope. Continuous and discontinuous precipitation of the α ′ -A13(Li x Hf1-x ) phase occurred in the Al-1.9 wt% Li-1.6 wt% Hf alloy during solution treatment at 450 ° C [79]. The α ′ -A13(Li x Hf1-x ) phase maintained a stable, fine distribution during solution treatment and controlled the strength of the alloy, while formed δ ′ -A13Li during aging homogeneously distributes within the matrix and at the α ′ -matrix interface and envelopes of the α ′ -phase [81].
Hallem [60] has investigated the evolution of the morphology and grain size in the dilute cast Al-Hf alloys. The equiaxed structure was observed in the Al-0.17 wt% Hf alloy, while the island grains and tendencies to the feather-shaped pattern typical for twinned columnar grains (TCGs) were observed in the Al-0.95 wt% Hf alloy. It was found that addition of Hf could refine grains. Table 6 shows that the grain size decreases from 917 to 475 μ m when the Hf content increases from 0.17 to 0.95 wt%. The conclusions are in agreement with the investigations conducted by Hori and Furushiro [50]. The remarkable grain refinement took place when the Hf content exceeded 4% (Fig. 14). In this case, the primary Al3Hf-particles first formed acted as the nucleation sites for α -Al [43, 49]. In addition, Brodova et al. [48] have investigated the microstructure of the Al-1.4 wt% Hf alloy which was carried out with different overheating conditions of the initial melt above liquidus and various cooling rates. It was found that the increase of both overheating and cooling rate may efficiently decrease the mean grain size as shown in Fig. 15.
| Table 6 Grain size measurements of the as-cast alloys [60] |
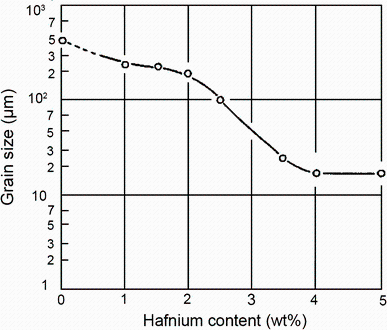 | Fig. 14 Relation between grain size and Hf content in the cast alloy |
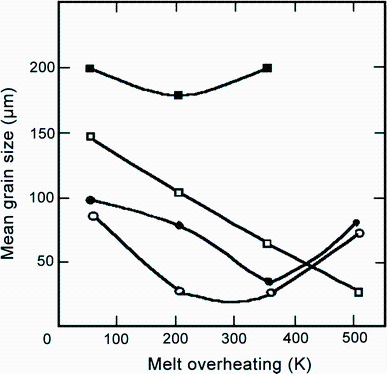 | Fig. 15 Mean grain size in castings obtained at various cooling rates versus initial melt overheating above liquidus.Filled square V = 2 × 102 K/s, open square V = 4× 103 K/s, filled circle V = 104 K/s, open circleV = 2 × 104 K/s |
Lattice mismatch is an important factor that can be used to predict coherency, or lack of it, between matrix and precipitates. We follow the scheme of Zedalis and Fine [73] for evaluating the lattices mismatch between the Al3X intermetallics and aluminum. The overall mismatch δ was defined by the following equation, given by Zedalis and Fine [73] who studied the lattice parameter variation of Al3(Ti, V, Zr, Hf) in Al-2 at.% (Ti, V, Zr, Hf) alloys
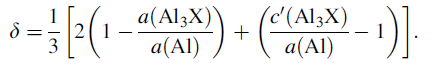
Norman and Tsakiropoulos [43] reported that hafnium increased the lattice parameter of α -Al at a rate of 0.0003 nm per wt% Hf, which is similar to the results from Hori et al. [29] (Fig. 16). Al3Hf with the L12structure has the lowest the mismatch δ with the Al matrix compared with the mechanically alloyed Al3Ti and Al3Zr which was observed by Srinivasan et al. [42]. Harada and Dunand [64] have reported that the partial substitution of Sc-atoms by Hf leaded to a decrease in the lattice mismatch δ between matrix and precipitates. The lattice parameter of the L12 solid solution decreased linearly with increasing concentration of Group IVA metals including Hf, and this linear concentration dependence of the lattice parameter was found to correlate to the atomic size mismatch between Sc and the transition metal.
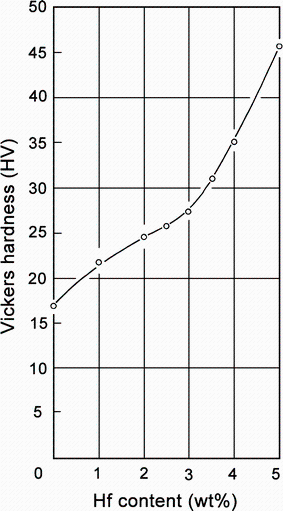
Hori et al. [49] have studied the effect of hafnium content on Vickers hardness in Al-0-5 wt% Hf cast alloys. The increase of the hafnium content in the alloys generally increased the Vickers hardness of the alloy. When the Hf content exceeded 3.5 wt%, increase of hardness became even fast (Fig. 17). This may be due to the combination of grain refinement with the solid-solution hardening. Norman and Tsakiropoulo [43] also observed the microhardness improvement by 167.58 MPa per wt% Hf (17.1 kg/mm2 per wt% Hf) by hafnium addition. The microstructure of the ternary trialuminide Al3(Sc1-yX y ), where X is a transition metal from Groups IIIA (Y), IVA(Ti, Zr, Hf) or VA(V, Nb, Ta), was investigated by Harada and Dunand [64]. The conclusions were that the microhardness of the L12-type Al3(Sc1-yX y ) phase increased linearly with increasing concentration of ternary elements. Dependence of hardness to concentration was strongest for Group VA metals and weakest for Group IVA metals.
Electrical conductivity measurement is a suitable way to obtain information about the elements in solid solution and the occurrence of precipitation reactions during annealing. Seth and Woods [83] gave the description of the relationship between electrical conductivity and the solid-solution content for the investigated alloys by neglecting the temperature-dependent term in the Matthiessens rule:
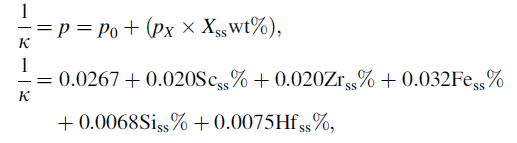
where κ is the electrical conductivity (MS/m), p is the total resistivity (μ Ω cm/wt%), p0 is the resistivity of the base material (Al-99.999 wt%), p X is the contribution from each alloying element in solid solution and Scss%, Zrss% Fess%, Siss% and Hfss% represent the weight percent of Sc, Zr, Fe, Si and Hf in solid solution, respectively.
Rangel-Ortiz et al. [76] found that Hf was present as an intermetallic precipitate, improving the hardness of the as-cast Al-1.65 wt% Li-0.85 wt% Hf alloy. Hallem [60] has investigated the effect of Hf in solid solution on the conductivity. By using the equation above, pHf was found to be approximately 0.75 μ Ω cm/wt%. This value has subsequently been used to calculate the amount of Hf in solid solution in the Al-Hf alloys containing Si, Fe, Sc and Zr. Table 7 gives the measured and calculated values of resistivity in the alloys. It seems that all the alloying elements go into solid solution. However, some primary particles are still observed during the microstructure investigations.
| Table 7 Calculated amounts of elements in solid solution from conductivity measurements in Al-Hf-(Sc)-(Zr) alloys [60] |
A systematic creep study was undertaken for the binary intermetallic Al3Sc and the ternary single-phase intermetallic Al3(Sc0.74Hf0.26) by Harada and Dunand [84]. The activation energy of Al3(Sc0.74Hf0.26) for creep deformation increased apparently. Jia and Arnberg [61] found a high density of Si2Hf precipitates with the “ nanobelt” morphology after an appropriate heat treatment in an Al-7 wt% Si-0.3 wt% Mg-0.5 wt% Hf-0.2 wt% Y alloy. The unpublished experimental results show that such precipitate could improve evidently the high-temperature creep resistance, but less influence on hardness and strength of the material.
Addition of hafnium in aluminum alloys has considerable effect on the microstructure and properties of the alloys which depends on the existing form of Hf in aluminum alloys. Because of solubility of Hf as a function of temperature in aluminum, the primary and/or secondary Hf-containing particles could be formed during solidifying process or subsequent annealing treatment. The secondary Al3Hf particles may precipitate discontinuously or continuously and the phase transformation from a metastable L12 cubic Al3Hf to equilibrium D022 or D023 tetragonal Al3Hf can occur, which are greatly related to preparation processes of the alloy and heat treatments. Some coexisting elements in the Al-Hf alloys could influence the precipitation behavior and phase composition. Addition of Hf together with Sc and/or Zr will form the complex Al3(Sc, Hf), Al3(Zr, Hf) or Al3(Sc, Zr, Hf) precipitates, which are more stable and show higher density, comparing to the individual Al3Sc or Al3Zr precipitate. This is due to slow diffusivity of Hf and decreased solubility of Hf, Sc and Zr in aluminum by co-addition. Amount of Si have great influence on the formation Hf-containing phases. Less Si could promote the formation of Al3Hf phase, and the AlSiHf phase will be formed when the Si content is over 0.15 wt%. Interestingly, the Si-Hf phases can be formed in aluminum alloys with high Si (7 wt%), which exhibits even high-temperature stability and high density. How to tailor these Hf-containing phases is very important for improving the properties of aluminum alloys at evaluated temperature, which will be an interesting point for future work.
The authors have declared that no competing interests exist.
作者声明: 无竞争性利益关系
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|