In this work, pure nickel and Ni-based nanocomposite coatings (Ni-Al2O3, Ni-SiC and Ni-ZrO2) were produced on steel substrate by using pulse electrodeposition technique. The industrial performance tests were conducted to evaluate the wear resistance, corrosion resistance, adhesion strength and wettability behaviour of newly developed coatings. Rolling contact ball-on-disc tribometer was used to assess anti-wear behaviour of these coatings under water-lubricated contacts. The results showed that the wear- and corrosion resistance properties of nickel alumina and Ni-SiC composite coatings significantly improved than that of pure Ni and Ni-ZrO2 coatings. The adhesion and wettability results of Ni-Al2O3 composite showed better performance when compared to the rest of the coatings. The effects of incorporating nanoparticles on the surface microstructure, interface adhesion and distribution of the particles were also investigated. The coatings were characterized by using scanning electron microscopy, X-ray diffraction analysis and 3D white light interferometry. The wear failure behaviour of these coatings was further examined by post-test surface observation under optical microscope.
Material’ s surfaces or components have limited life due to the mechanical interactions of components within the system and/or chemical or electrochemical influences of operating environment. During the last decade, extensive work has been completed in the field of electrodeposition coating technique, especially with a focus on wear and corrosion-resistant coatings, self-lubricating systems and dispersion-strengthened coatings [1, 2, 3, 4, 5, 6].
Recently, Gü l et al. [7, 8] compared the effect of current density on the tribological performance of Ni-Al2O3 composite coatings deposited using DC and pulse current techniques. The surface morphology results showed that the composite coatings produced from PC deposition contain a higher-loaded percentage of incorporating particles and better particle distributions. The wear results demonstrated that in DC-plated coatings the increasing current density contributed to reduced wear rate. However, in case of PC-plated composite coating, insignificant effect was observed on the wear rate with increasing current density. Researchers have also reported the optimum values for incorporating particles [9] and sodium dodecylsulfate (SDS) [10] concentrations in the electrolyte of Ni-SiC and Ni-WC composite coatings, respectively. These values are 20 g/L for particle concentration and 0.10 g/L for SDS in order to achieve distinctly improvement of the mechanical and wear-resistant properties. The effect of ultrasonic treatment on incorporation of zirconia particles into a nickel matrix was investigated by Beltowska-Lehman et al. [11]. It was found that the Ni-W/ZrO2 composite coatings exhibit uniform particle distribution, enhanced content of the particles at lower ultrasonic treatment and consequently improved the mechanical properties achieved.
Considerable studies were conducted to examine the tribological performance of nanocomposite coatings embedded with nanoparticles into nickel as matrix [12, 13, 14, 15, 16, 17]. Nanoparticles present unique and novel properties, typically when their size is below 100 nm. The effect of nanosized Al2O3 composite with nickel matrix on anti-corrosion properties of nickel coating was studied by Zhou et al. [18]. They demonstrate that the incorporation of Al2O3 nanoparticles improves the corrosion resistance by reducing the porosity of nickel coatings and also improves the abrasion resistance due to better dispersion of nano-Al2O3 as compared to micro-Al2O3. The microhardness and anti-wear resistance performance of electrodeposited nanocomposite coatings was studied by Borkar et al. [19]. They showed that the increasing weight content of the nanoparticles into the electrolyte bath (up to 40 g/L) significantly improves the microhardness and the wear resistance of the nickel coating due to the enhanced strengthening effect of the nanoparticles into nanocomposite coatings. They also investigate the effect of plating conditions and concluded that the pulse current and pulse reverse current enhanced the weight percentage of reinforcement of the nanoparticles into the coating better as compared to direct current deposition.
The influence of pulse frequency on the sliding wear property of the nanocomposite coatings was examined by Chen et al. [20]. They indicated that the volume fraction of alumina particles in coatings increased with the increase in pulse frequency and the wear behaviour of coating depends on reinforcement content of the particles into coating rather than pulse frequency. They demonstrated that the anti-wear properties of electrodeposited coatings under various pulse frequencies behave significantly different under dry and oil-lubricated environments.
Previous studies [21, 22, 23, 24, 25] investigated the rolling contact fatigue behaviour of thermal plasma sprayed coated rolling elements under various tribological conditions. They indicated that the coating failure mechanisms under rolling contact based on two kinds of failure modes: the surface wear and coating delamination. Additionally, Khan et al. [26, 27, 28, 29] studied the failure mechanisms of hybrid ceramic rolling contact bearing elements in rolling or rolling/sliding contact with conventional oil and saturated liquid refrigerant lubrication.
The tribological performance of electrodeposited nickel-based coatings was studied by various researchers as reported above. However, the tribological performance of electrodeposited nanocomposite coatings in rolling contact subject to water-lubricated regime has been left obscure. Earlier investigations have utilized pin-on-disc or ball-on-disc sliding tribotest conditions under dry or oil-lubricated environments.
In current work, a high-speed microprocessor rotary tribometer (HSMRT) was used to simulate industrial applications in terms of rolling contacts. The pulse current electrodeposition technique was utilized to electrodeposit pure nickel as a mean of benchmarking, and nickel composite coatings incorporating nanosized alumina, SiC and ZrO2 particles. These coatings were investigated in terms of their tribological and mechanical properties, coatings-substrate interface adhesion strength, wettability behaviour and surface free energies. The pure nickel and nickel alumina composite coatings have been extended from our previously published work in which [30] the influences of the different ionic strength of electrolyte were studied.
The surface energy of the coatings plays an important role in the substrate-coating interface adhesion and adhesion resistance on counter body in tribological applications. Therefore, the effect of various embedded nanoparticles into electrodeposited nanocomposite coatings subject to surface energy and wettability behaviour was also investigated in this study [31].
All coatings, pure nickel, Ni-Al2O3, Ni-SiC and Ni-ZrO2, were pulse electrodeposited with the expected thickness of ~10 µ m over steel substrate. The electrolyte composition consists of NiSO4· 6H2O (265 g/L), NiCl2· 6H2O (48 g/L) and H3BO3 (31 g/L). For composite coatings, the nanoparticles (~50 nm) with an amount of 20 g/L were ultrasonically dispersed in the electrolyte. The electrodeposition process parameters were kept constant as current density (3 A/dm2) and pulse on-off time (20-80 ms) with a duty cycle of 20%. A high-quality nickel sheet was used as an anode, and a steel circular disc of 80 mm diameter and 8.20 mm thick was used as a cathode.
Scanning electron microscopy (JSM-6010, JEOL) was used to analyse the microstructure of the deposits. The weight percentages of embedded particles were calculated by the energy-dispersive X-ray spectroscopy (EDS). X-ray diffraction (XRD) analysis was performed by using a D8 Advance (Bruker) with 2θ range of 20° -100° and with a step increment of 0.02° . For coating-substrate interfacial adhesion analysis, the specimens were embedded by using ATM OPAL 460 equipment as shown in Fig.1. The hardness and elastic modulus of the coatings were measured by using CSM microindentation tester (MHT) at a loading force of 300 mN.
The wear resistance tests conducted by using a unidirectional ball-on-disc tribometer in distilled water at a rolling speed of 1 m/s and normal load of 45 N. The corresponding Hertzian contact pressure [32] can be estimated to be 1.75 GPa. The tribometer consists of an upper plated specimen and lower three balls (100Cr6) spaced at 120° . 3D profile of one sample’ s wear track is shown in Fig.1. The wear volume was calculated as V=AL, where A is the cross-sectional area of worn scar in mm2 and L is the length of the worn scar in millmetres. A specific wear rate is determined as v=V/(Fl), where F is the load in N and l is the rolling distance in metres [33]. All wear tests were conducted at least two times to ensure repeatability and minimize uncertainty.
The electrochemical measurements were taken by using a system of three electrodes. The deposit part was used as a working electrode, and a platinum wire was used as counter electrode. The reference electrode was an Ag/AgCl. The electrochemical results were obtained at opening circuit potential (OCP) with scanning rate of 0.001 V/s.
The scratch tests were conducted DIN EN 1071-3 standard method by using a CSM REVETEST machine. The scratch was made with a length of 10 mm on each type of the coating with a sliding speed of 10 mm/min and increasing load from 0 to 100 N/min. The critical load value (Lc) is defined as the load value at which the first mode of failure was observed. The surface free energy was conducted using Owens and Wendt (OWRK) method considering water and diiodomethane as reference liquids of known surface energies [34].
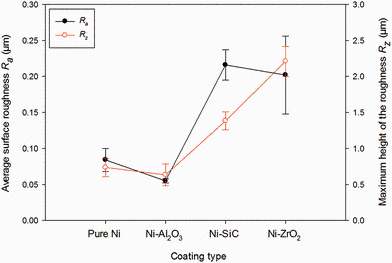
Pure nickel and nickel composite coatings were electrodeposited under same pulse parameters exhibit shiny grey appearance, surface uniformity and good adherence to the mild steel substrate. The micrographs of pulse electrodeposited pure nickel and nickel composite of nanosized particles of alumina, silicon carbide and zirconium are shown in Fig.2. All the pulsed deposited coatings exhibit compact fine grain size. However, the grain size of co-deposited Ni-SiC exhibits the highest grain size when compared to the rest of the coatings which causes higher surface roughness parameters (Ra ~ 0.21 µ m; Rz ~ 1.38 µ m). The variation in average surface roughness (Ra) and the maximum height of the roughness (Rz ) values for deposited coatings are shown in Fig.3. The presence of agglomerated particles and pores on the Ni-ZrO2 coatings resulted in higher surface roughness values (Ra ~ 0.20 µ m; Rz ~ 2.2 µ m). The surface roughness values were minimum (Ra ~ 0.05 µ m; Rz ~ 0.63 µ m) for Ni-alumina followed by pure nickel (Ra ~ 0.08 µ m; Rz ~ 0.73 µ m) due to finer surface morphology. The cross-sectional SEM micrographs of incorporating nanoparticle distributions of composite coatings are shown in Fig.4.
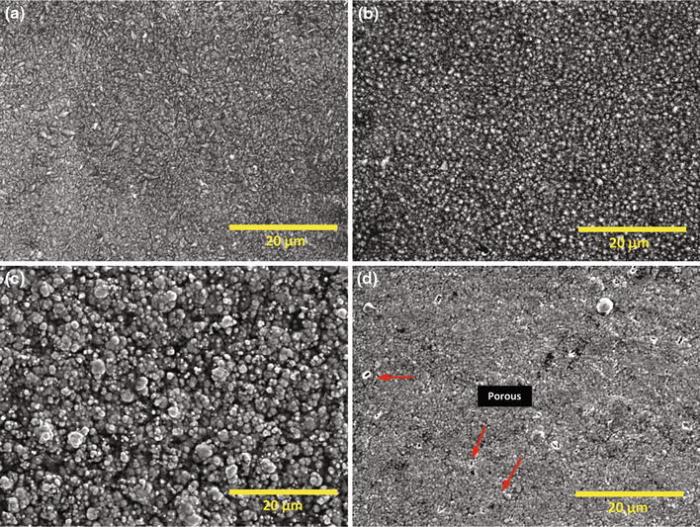 | Fig.2 Surface morphologies of pulse electrodeposited nickel-based composite coatings: a pure nickel, b Ni-Al2O3, c Ni-SiC, d Ni-ZrO2 |
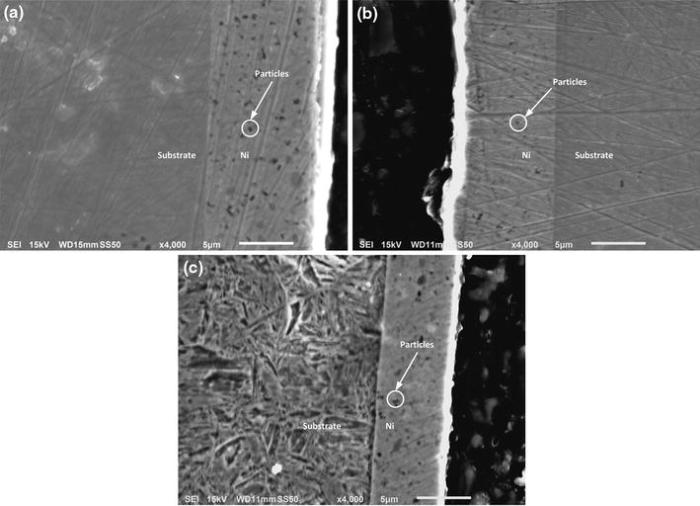 | Fig.4 Cross-sectional SEM micrographs of incorporated nanoparticle distributions in the nickel-based composite coatings: a Ni-Al2O3, b Ni-SiC, c Ni-ZrO2 |
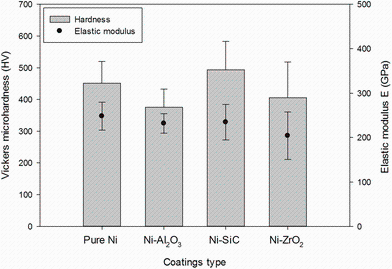
The weight percentage of reinforcement content was calculated through energy-dispersive spectroscopy to be 2.5-3 for the nickel composite coatings. The microhardness and elastic modulus properties of the nickel and nickel composite coatings, electrodeposited under same pulse conditions, but with different types of incorporated particles are shown in Fig.5. There was no significant difference observed in the hardness values between all types of the coatings which contradicts the previous finding. Previously, it has been reported that the hardness of the composite coatings was significantly improved due to the strengthening effects of incorporated hard particles. However, the reinforcement content of hard particles in that study was (8-12) wt% [19]. Generally, the increase in the weight percentage of incorporated hard particles into nickel matrix results in enhanced mechanical properties. However, in practice the hardness to elastic modulus ratio (H/E) is an important parameter in predicting the better mechanical properties of materials [11]. Thus, lower hardness of Ni-Al2O3 (375HV) and Ni-ZrO2 (405HV) composites than pure nickel coating can be attributed to lower plasticity index ratio (H/E) and porous surface morphology, respectively. As shown in Fig.5, the Ni-SiC coating exhibits the higher hardness to elastic modulus (H/E) ratio, which is an indication of good wear resistance property. This is because that H/E ratio is a better parameter for predicting wear resistance property than hardness alone [35].
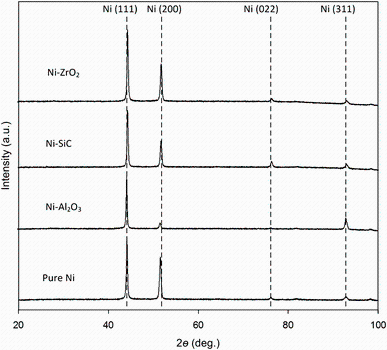
The X-ray diffraction (XRD) patterns for pure nickel and nickel-based composite (Ni-Al2O3, Ni-SiC and Ni-ZrO2) coatings are shown in Fig.6. For all the coatings, the XRD patterns show typical high peaks for (111) and (200) crystal planes of nickel and also evidenced by other researchers [7, 9, 11]. There are visible changes in orientation between the four samples; in particular, the changes affect the (311) reflection, which grows more intense for the Ni-alumina where the (002) grows less intense. For the Ni-SiC, there is a visible more intense orientation indicated by the (022) reflection. Note that due to very low reinforcement content percentage of nanoparticles, the corresponding peaks could not be resolved [19]. The crystalline grain sizes were calculated by using XRD patterns and observed less than 50 nm for all coatings: pure Ni=39 nm, Ni-alumina=33 nm, Ni-SiC=34 nm and Ni-ZrO2=30 nm. The average grain size slightly reduced with the addition of the nanoparticles into a nickel matrix. Many studies have shown that the reinforcement of the nanosized particles into Ni matrix reduces the average grain size of Ni matrix [8, 10, 36].
The wear rates of pure nickel and nickel composite coatings in water-lubricated rolling contacts are shown in Fig.7. The Ni-SiC showed the minimum wear rate followed by Ni-Al2O3 in water-lubricated contacts. There was no significant difference in wear rates of Ni-ZrO2 and pure nickel electrodeposited coatings. For all coatings, the two-body abrasive wear and plastic deformation were observed due to the higher hardness of counter steel balls than coatings as shown in Fig.1. However, relatively severe plastic deformation in a form of continuous wide grooves in the case of pure nickel and nickel-ZrO2 was an indication of poor wear resistance property. Post-test surface examinations of worn wear tracks were further investigated through SEM and are shown in Fig.8a-d and magnified view in Fig.8e-h. Similar severe plastic deformation appearances of the pure nickel and Ni-ZrO2 led to the smoothening of the surface as shown in Fig.8e, h, while, in the analysis of worn Ni-Al2O3 and Ni-SiC, the surfaces microploughing and delamination wear mechanism were observed, respectively, as shown in Fig.8f, g. It may relate to SiC and Al2O3 particles pulling out of the matrix and contribute to three-body abrasion wear [33]. However, lower wear rate in Ni-SiC and Ni-Al2O3 composites indicates that SiC and Al2O3 hard particles are restricting severe plastic deformation in worn surfaces.
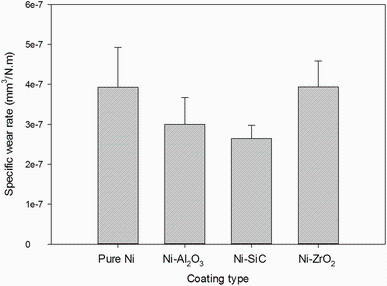 | Fig.7 Comparison of the wear rates of pure nickel and nickel composite coatings in water-lubricated rolling contacts |
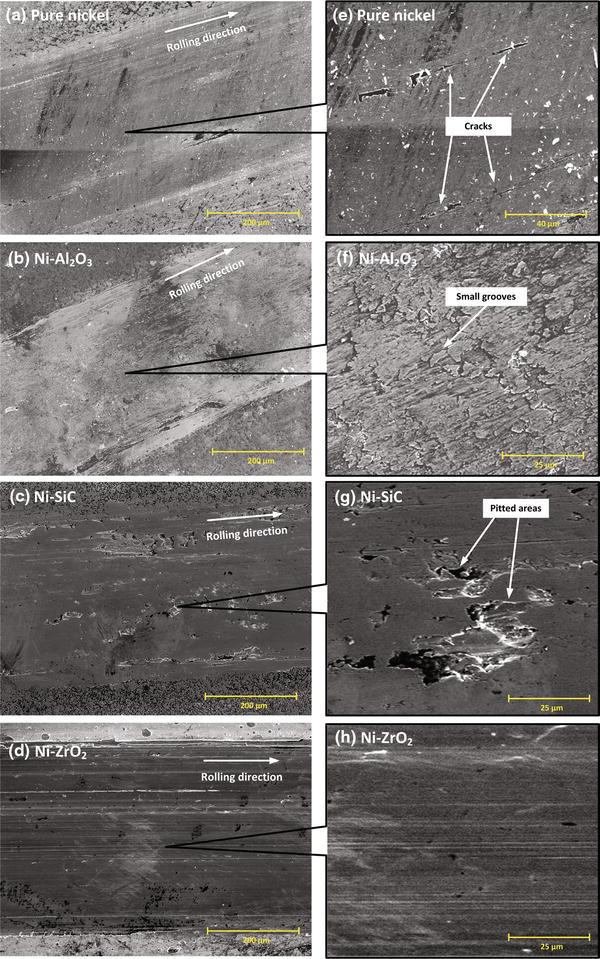 | Fig.8 SEM micrographs of worn surfaces of pure nickel and nickel composite coatings in water-lubricated rolling contacts; overall a-c, magnified d-f views |
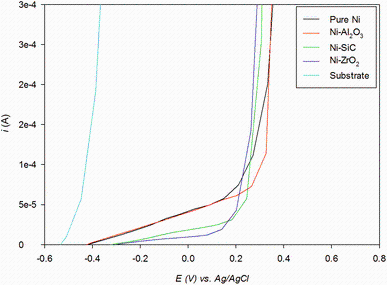
The potentiodynamic curves for the steel substrate and electrodeposited coatings are shown in Fig.9. As can be seen, the corrosion resistance potential for deposited coating is significantly higher than the smooth substrate surface. In comparison, the Ni-Al2O3 exhibits the higher corrosion resistance potential followed by pure nickel. This can be attributed to finer grain structure with the addition of Al2O3 particles and validates the previous findings [37, 38]. It is interesting to observe that the corrosion potential of Ni-SiC and Ni-ZrO2 is lower than pure nickel and exhibits similar behaviour. The lower corrosion potential behaviour in nickel composite coatings incorporating SiC and ZrO2 can be because of higher surface roughness (Ra) and maximum height of the roughness (Rz ) parameter values. The roughness valleys in these coatings can provide weak points for penetration of aggressive corrosive solution towards substrate and consequently cause lower pitting potential.
The adhesion performance of all coatings was investigated to evaluate their suitability in industrial application. The adhesion critical load values (Lc) measured in scratch tests for pure nickel and nickel composite coatings are compared in Fig.10. All coatings except Ni-ZrO2 showed adhesion strength above the critical load of 30 N which is generally required as a minimum critical load value for industrial applications. Nickel composite of alumina nanoparticles showed the maximum Lc value of 79 N and lowest Lc value of 28 N and was observed in Ni-ZrO2 composite coatings. Note that these coatings were deposited on mild steel substrate without any intermediate coating which can further improve adhesion strength. Coatings failure mechanism in scratch testing was observed through microscopy and is shown in Fig.11.
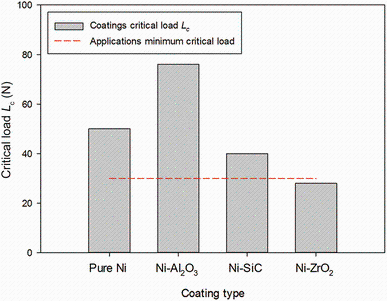 | Fig.10 Critical loads for different types of nickel and nickel composite coatings according to DIN EN 1071-3 |
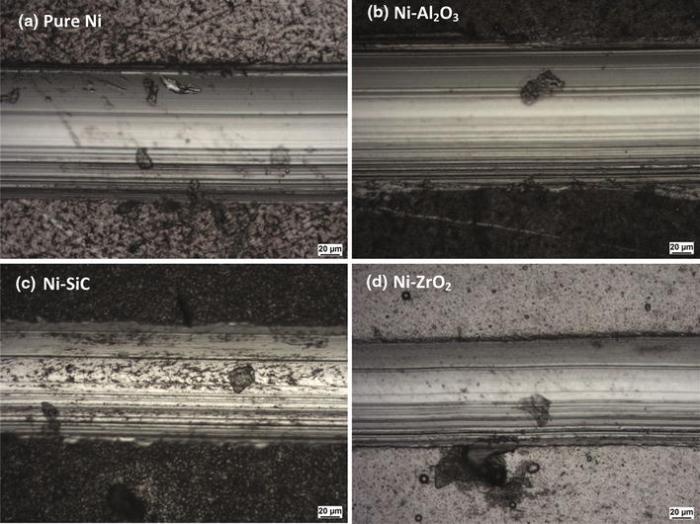 | Fig.11 Optical microscopic examinations for failure mechanism according to DIN EN 1071-3 for nickel and nickel composite coatings |
Figure 12 shows the polar and dispersive component of the surface free energy and water contact angle measurement results of all coatings. In all coatings, the higher dispersive component share reveals that the coatings mainly possess covalent bonds. Their disperse shares vary from 29 to 36 mN/m, and polar shares vary from 2 to 16 mN/m. It is noticeable that no significant differences were observed in wettability behaviour between pure nickel and nickel composite coating. However, the polar component of Ni-Al2O3 composite coating was remarkably reduced by addition of nanosized alumina particles. The reduced polar component in nickel alumina composite coatings results into the higher contact angle of water as compared to the rest of the coatings. The surfaces become less hydrophilic with reducing polar shares due to reduction in dipole interactions [39]. Furthermore, it is noticeable that lower surface energy of Ni-Al2O3 coating exhibits the highest adhesion to the substrate which is contrasting to previous investigations [31]. Their studies concluded that the high adhesion can be achieved with increasing high surface energies. However, they used PVD method for coating development which may be the reason for the present contradiction.
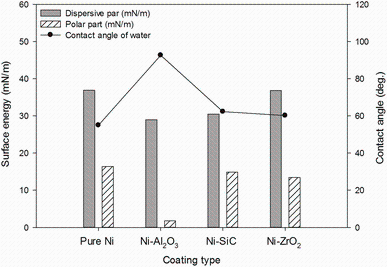 | Fig.12 The comparison of the surface energy and changing wettability behaviour of the pure nickel and nickel composite coatings |
(1)The wear-resistant results show that the Ni-SiC and Ni-Al2O3 provide a better wear resistance performance than pure nickel and Ni-ZrO2 coatings.
(2)Nickel composite of nanosized alumina exhibits the maximum corrosion resistance potential followed by pure nickel, Ni-SiC and Ni-ZrO2.
(3)In scratch test, the Ni-alumina composite showed a better adhesion strength on a substrate as compared to rest of the coatings.
(4)The low surface energy and in return the higher water contact angle of nickel--alumina composite coating predict a better corrosion resistance performance in water-lubricated contacts.
(5)The cross-sectional images of the coatings show that all the coatings are uniform and well adhere to the substrate without cracks at the coating-substrate interface.
The authors would like to acknowledge Schaeffler Technologies GmbH & Co. KG (Germany) and Bournemouth University (UK) for financial and in-kind support. The authors also thank Dr Lorna from the Experimental Techniques Centre, Brunel University, for her assistance with XRD analysis.
The authors have declared that no competing interests exist.
作者声明: 无竞争性利益关系
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|